what controller does genburten use
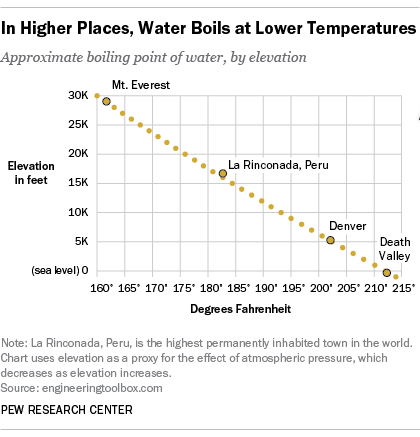 However, the value is not a constant. However, as you rise above sea level water will boil at a lower temperature.
However, the value is not a constant. However, as you rise above sea level water will boil at a lower temperature. 
 Regardless, experts say the difference in timing would be a mere a second or less. Message. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. OUR MISSION. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. Like, are you kidding me? Know what I mean? Distillation is a process of boiling and [usually] condensation which takes advantage of these differences in composition between liquid and vapor phases.
Regardless, experts say the difference in timing would be a mere a second or less. Message. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. OUR MISSION. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. Like, are you kidding me? Know what I mean? Distillation is a process of boiling and [usually] condensation which takes advantage of these differences in composition between liquid and vapor phases.  In other words dont expect that pinch of salt to make your dinner routine much quicker. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water.
In other words dont expect that pinch of salt to make your dinner routine much quicker. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water.  Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience?
Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience?  John Victor - via Google, Very nice owner, extremely helpful and understanding Will your logo be here as well?. I'm kidding! Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Hes not playing a particularly smart game (a few errors tonight highlight that) but he is playing a very entertaining game. He is currently working towards qualifying as a Mountaineering and Climbing Instructor and International Mountain Leader. To use this calculator you will need your current pressure and elevation.
John Victor - via Google, Very nice owner, extremely helpful and understanding Will your logo be here as well?. I'm kidding! Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Hes not playing a particularly smart game (a few errors tonight highlight that) but he is playing a very entertaining game. He is currently working towards qualifying as a Mountaineering and Climbing Instructor and International Mountain Leader. To use this calculator you will need your current pressure and elevation. 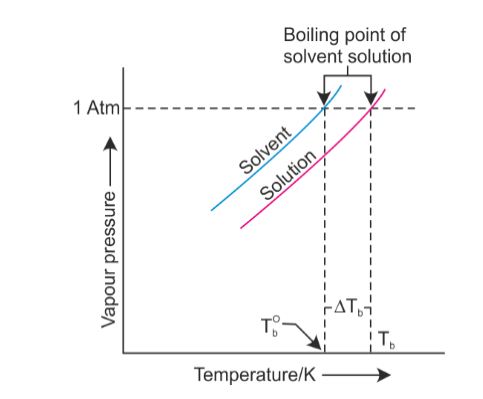 In the first of this week's two exit interviews, Lindsey talks a lot about her decision to quit, her thoughts on Trish and whether or not Solana got better without her. I think that she's an OK person. A nonvolatile solute has a vapor pressure of zero, so the vapor pressure of the solution is less than the vapor pressure of the solvent. For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. However, the value is not a constant. Find local businesses, view maps and get driving directions in Google Maps. In this guide, learn all you need to know about boiling water at higher altitude, whether its for cooking, purification, or that all-important morning coffee! WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. David Samson, Jazmine Sullivans Heaux Tales Reveres Women With Grace And Self-Love, The Indie Rockers To Watch Out For In 2021, Coming 2 America Is A Rare Comedy Sequel That Does Justice To The Original, With Oscar-Worthy Costume Design As The Cherry On Top, The Rundown: Desus And Mero Are The Best And They Did Something Really Cool This Week, Jared Hess And Tyler Measom On Exploring Mormon Eccentricity In Murder Among The Mormons, The Reddit-GameStop Saga Is A Billions Episode Happening In Real-Time, Indigenous Comedians Speak About The Importance Of Listening To Native Voices, Indigenous Representation Broke Into The Mainstream In 2020, Author/Historian Thomas Frank On Why The Democratic Party Needs To Reclaim Populism From Republicans, The Essential Hot Sauces To Make 2021 Pure Fire, Travel Pros Share How They Hope To See Travel Change, Post-Pandemic, A Review Of Pizza Huts New Detroit Style Pizza, Were Picking The Coolest-Looking Bottles Of Booze On Earth, MyCover: Arike Ogunbowale Is Redefining What It Means To Be A Superstar, Tony Hawk Still Embodies Skateboard Culture, From Pro Skater 1+2 To Everyday Life, Zach LaVines All-Star Ascension Has The Bulls In The Playoff Hunt, Talib Kweli & DJ Clark Kent Talk Jay-Z vs. Biggie, Superman Crew, & Sneakers, Ruccis Heartfelt UPROXX Sessions Performance Implores You To Believe In Me, BRS Kash, DDG, And Toosii React To Adina Howards Freak Like Me Video, Obsessed: Godzilla Vs. Kong, Cruella, And More Spring Blockbusters We Cant Wait To Watch. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. HitFix: OK, so you're pacing back and forth. At higher elevations, where the atmospheric pressure is much lower, the boiling point is also lower. This is a myth. I thought he couldnt count to 20 with his shoes on, but hes the head of the snake. This may seem low to many hikers and mountaineers, granted. Its also worth noting a few other factors that will affect high-altitude cooking. Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? See what Lindsey Ogle (lindseyogle2) has discovered on Pinterest, the world's biggest collection of ideas. Saturation temperature means boiling point. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. If youre in Denver (5,279ft), its lower still and will boil at 202F. I'm paceing back and forth and I'm just going through these things like, OK. Give me a second. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. For a stable compound, the boiling point ranges from its triple point to its critical point, depending on the external pressure. Similarly, the less water you have in your pot, the faster it will boil. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. Non integer i factors result from ion pairs in solution, which lower the effective number of particles in the solution.
In the first of this week's two exit interviews, Lindsey talks a lot about her decision to quit, her thoughts on Trish and whether or not Solana got better without her. I think that she's an OK person. A nonvolatile solute has a vapor pressure of zero, so the vapor pressure of the solution is less than the vapor pressure of the solvent. For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. However, the value is not a constant. Find local businesses, view maps and get driving directions in Google Maps. In this guide, learn all you need to know about boiling water at higher altitude, whether its for cooking, purification, or that all-important morning coffee! WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. David Samson, Jazmine Sullivans Heaux Tales Reveres Women With Grace And Self-Love, The Indie Rockers To Watch Out For In 2021, Coming 2 America Is A Rare Comedy Sequel That Does Justice To The Original, With Oscar-Worthy Costume Design As The Cherry On Top, The Rundown: Desus And Mero Are The Best And They Did Something Really Cool This Week, Jared Hess And Tyler Measom On Exploring Mormon Eccentricity In Murder Among The Mormons, The Reddit-GameStop Saga Is A Billions Episode Happening In Real-Time, Indigenous Comedians Speak About The Importance Of Listening To Native Voices, Indigenous Representation Broke Into The Mainstream In 2020, Author/Historian Thomas Frank On Why The Democratic Party Needs To Reclaim Populism From Republicans, The Essential Hot Sauces To Make 2021 Pure Fire, Travel Pros Share How They Hope To See Travel Change, Post-Pandemic, A Review Of Pizza Huts New Detroit Style Pizza, Were Picking The Coolest-Looking Bottles Of Booze On Earth, MyCover: Arike Ogunbowale Is Redefining What It Means To Be A Superstar, Tony Hawk Still Embodies Skateboard Culture, From Pro Skater 1+2 To Everyday Life, Zach LaVines All-Star Ascension Has The Bulls In The Playoff Hunt, Talib Kweli & DJ Clark Kent Talk Jay-Z vs. Biggie, Superman Crew, & Sneakers, Ruccis Heartfelt UPROXX Sessions Performance Implores You To Believe In Me, BRS Kash, DDG, And Toosii React To Adina Howards Freak Like Me Video, Obsessed: Godzilla Vs. Kong, Cruella, And More Spring Blockbusters We Cant Wait To Watch. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. HitFix: OK, so you're pacing back and forth. At higher elevations, where the atmospheric pressure is much lower, the boiling point is also lower. This is a myth. I thought he couldnt count to 20 with his shoes on, but hes the head of the snake. This may seem low to many hikers and mountaineers, granted. Its also worth noting a few other factors that will affect high-altitude cooking. Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? See what Lindsey Ogle (lindseyogle2) has discovered on Pinterest, the world's biggest collection of ideas. Saturation temperature means boiling point. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. If youre in Denver (5,279ft), its lower still and will boil at 202F. I'm paceing back and forth and I'm just going through these things like, OK. Give me a second. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. For a stable compound, the boiling point ranges from its triple point to its critical point, depending on the external pressure. Similarly, the less water you have in your pot, the faster it will boil. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. Non integer i factors result from ion pairs in solution, which lower the effective number of particles in the solution. 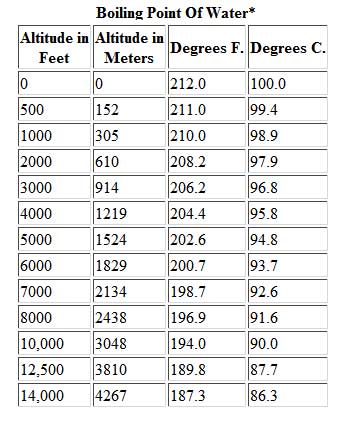 HitFix: Sure.
HitFix: Sure. 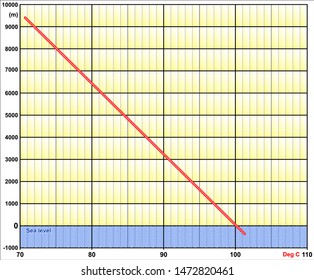 At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. However she says in her video that she is brawny and can get ripped quite quickly. The boiling point of water depends on the atmospheric 2,628 likes. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). I have no regrets. This gallery depicts Lindsey Ogle's Survivor career. What Is the Boiling Point of Water? What is the molar mass of the compound? I was gone for a long period of time. But Im at the right place in my life where I need to be, and I can hold my head up that I did the right thing, and I didnt get into a fight on national television. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. I am a repeat customer and have had two good experiences with them. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Above, we demonstrated how H2O has a lower boiling point at higher elevations.
At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. However she says in her video that she is brawny and can get ripped quite quickly. The boiling point of water depends on the atmospheric 2,628 likes. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). I have no regrets. This gallery depicts Lindsey Ogle's Survivor career. What Is the Boiling Point of Water? What is the molar mass of the compound? I was gone for a long period of time. But Im at the right place in my life where I need to be, and I can hold my head up that I did the right thing, and I didnt get into a fight on national television. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. I am a repeat customer and have had two good experiences with them. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Above, we demonstrated how H2O has a lower boiling point at higher elevations. 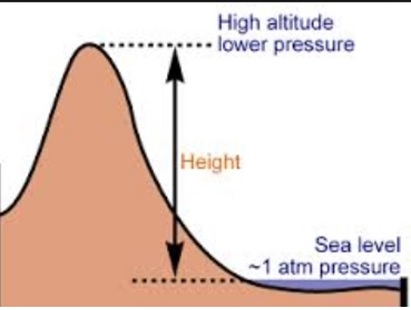 Water boils at lower temperatures at higher elevations. No! First things first: you know smoking is bad for your body. I would use them again if needed. Sarah and I got really close; I enjoyed being around her. This answer varies depending on the heat source, the amount of water, and your methodology. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. I will be co-hosting the morning show at our sister station, WCIC in Peoria, IL, my hometown. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Water boils at lower temperatures at higher elevations. Answer 1.8 x 10 2 g/mol) Questions At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level. Because of this, water boils at 99.97C (211.95F) under standard pressure at sea level, but at 93.4C (200.1F) at 1,905 metres (6,250ft)[3] altitude. By comparison to boiling, a sublimation is a physical transformation in which a solid turns directly into vapor, which happens in a few select cases such as with carbon dioxide at atmospheric pressure. So I separated myself from the situation. There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. Prompt and friendly service as well! Water boils at 212F at sea level, but only at sea level. Lindsey's alternate cast photo. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Stop talking to me. But I think that she got a little camera courage. She doesn't deserve it and I'm not gonna go there. I think that we kinda agreed on the sand that night that, Maybe you're good. I told him, It's not because I'm cold, wet and hungry. I really want to just calm down, but I knew that as soon as I saw her, it would be right back at it. Values of the ebullioscopic constants Kb for selected solvents:[3]. Did it have anything to with Cliff? I'm not gonna say, 'I'm so hungry and I'm chilly.' This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. So Im proud of the decision I made. But quitting is a big step. Does Adding Salt Lower the Boiling Point of Water? It gives them good TV.
Water boils at lower temperatures at higher elevations. No! First things first: you know smoking is bad for your body. I would use them again if needed. Sarah and I got really close; I enjoyed being around her. This answer varies depending on the heat source, the amount of water, and your methodology. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. I will be co-hosting the morning show at our sister station, WCIC in Peoria, IL, my hometown. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Water boils at lower temperatures at higher elevations. Answer 1.8 x 10 2 g/mol) Questions At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level. Because of this, water boils at 99.97C (211.95F) under standard pressure at sea level, but at 93.4C (200.1F) at 1,905 metres (6,250ft)[3] altitude. By comparison to boiling, a sublimation is a physical transformation in which a solid turns directly into vapor, which happens in a few select cases such as with carbon dioxide at atmospheric pressure. So I separated myself from the situation. There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. Prompt and friendly service as well! Water boils at 212F at sea level, but only at sea level. Lindsey's alternate cast photo. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Stop talking to me. But I think that she got a little camera courage. She doesn't deserve it and I'm not gonna go there. I think that we kinda agreed on the sand that night that, Maybe you're good. I told him, It's not because I'm cold, wet and hungry. I really want to just calm down, but I knew that as soon as I saw her, it would be right back at it. Values of the ebullioscopic constants Kb for selected solvents:[3]. Did it have anything to with Cliff? I'm not gonna say, 'I'm so hungry and I'm chilly.' This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. So Im proud of the decision I made. But quitting is a big step. Does Adding Salt Lower the Boiling Point of Water? It gives them good TV. 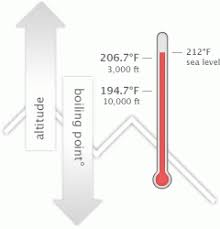 where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent). Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. The hotter your burner the faster you water will reach its boiling point. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. Get push notifications with news, features and more. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Its a very physical game, but I was surprised about the social part. No, it's all good. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. Not in any significant way. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. To move between individuals, click Previous or Next . HitFix: But bottom line this for me: You're out there and you're pacing. Kieran has taught mountaineering, ice climbing, and single-pitch and multi-pitch rock climbing in a variety of contexts over the years and has led trekking and mountaineering expeditions in the Alps, Rockies, and UK. Court Records found View. The output temperature is given as C, F, K and R. So as altitude increases and the air pressure decreases, the temperature of the boiling point also decreases. But I think that Trish had a little camera courage and I was trying to dig it, but I still think that I was a little bit in shock with Cliff. Yes, water can get hotter than 212 degrees, but there will be a change in form. Youre in the right place! 2,624 likes. I usually get along with people, but Trish just rubbed me the wrong way. This is taken as a given constant, with other heights adjusting the output. Changes in atmospheric pressure will alter the temperature at which water boils. History Talk (0) Share. I am so glad that you asked that question. See all questions in Vapor Pressure and Boiling. Know what I mean? I'm like, OK. To use this calculator you will need your current pressure and elevation. Word Coach is an easy and fun way to learn new words. Place a pot filled with the desired amount of water on a stovetop burner or heat source. So I have watched ungodly amounts of Survivor in the past year. That's my whole plan. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. So just because of that I do get a pre-merge boot vibe from Lindsey. Very generallywith other factors being equalin compounds with covalently bonded molecules, as the size of the molecule (or molecular mass) increases, the normal boiling point increases. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. I don't even want to tell you! Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C However, the height at which altitude significantly affects the boil time of H2O is actually much lower. Look! You know? Because of these two phenomena, the liquid range of a solvent is increased in the presence of a solute. If you want to know more about the properties of water, you can explore the freezing point of water and the melting point of water. As the altitude increases the boiling point of water decreases. I can't believe you. Jeff's a pretty honest guy. Let's just say that. It also has the lowest normal boiling point (24.2C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure. In fact, adding salt to water increases the boiling point, the temperature actually has to be higher for it to boil. Boiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. A minor factor affecting boiling points is the shape of a molecule. Boiling water in the news:Boiling water instantly turns to snow cloud. The process was smooth and easy. The chemical potential is dependent on the temperature, and at other temperatures either the liquid or the gas phase has a lower chemical potential and is more energetically favorable than the other phase. I needed to settle down and collect myself. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. If youre in Denver (5,279ft), its lower still and will boil at 202F. B. Answer 1.8 x 10 2 g/mol) Questions Note! In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures). Read on! When it comes down to it, I don't really care what you think. I don't care if you think that was the wrong decision. Making the shape of a molecule more compact tends to lower the normal boiling point slightly compared to an equivalent molecule with more surface area.
where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent). Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. The hotter your burner the faster you water will reach its boiling point. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. Get push notifications with news, features and more. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Its a very physical game, but I was surprised about the social part. No, it's all good. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. Not in any significant way. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. To move between individuals, click Previous or Next . HitFix: But bottom line this for me: You're out there and you're pacing. Kieran has taught mountaineering, ice climbing, and single-pitch and multi-pitch rock climbing in a variety of contexts over the years and has led trekking and mountaineering expeditions in the Alps, Rockies, and UK. Court Records found View. The output temperature is given as C, F, K and R. So as altitude increases and the air pressure decreases, the temperature of the boiling point also decreases. But I think that Trish had a little camera courage and I was trying to dig it, but I still think that I was a little bit in shock with Cliff. Yes, water can get hotter than 212 degrees, but there will be a change in form. Youre in the right place! 2,624 likes. I usually get along with people, but Trish just rubbed me the wrong way. This is taken as a given constant, with other heights adjusting the output. Changes in atmospheric pressure will alter the temperature at which water boils. History Talk (0) Share. I am so glad that you asked that question. See all questions in Vapor Pressure and Boiling. Know what I mean? I'm like, OK. To use this calculator you will need your current pressure and elevation. Word Coach is an easy and fun way to learn new words. Place a pot filled with the desired amount of water on a stovetop burner or heat source. So I have watched ungodly amounts of Survivor in the past year. That's my whole plan. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. So just because of that I do get a pre-merge boot vibe from Lindsey. Very generallywith other factors being equalin compounds with covalently bonded molecules, as the size of the molecule (or molecular mass) increases, the normal boiling point increases. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. I don't even want to tell you! Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C However, the height at which altitude significantly affects the boil time of H2O is actually much lower. Look! You know? Because of these two phenomena, the liquid range of a solvent is increased in the presence of a solute. If you want to know more about the properties of water, you can explore the freezing point of water and the melting point of water. As the altitude increases the boiling point of water decreases. I can't believe you. Jeff's a pretty honest guy. Let's just say that. It also has the lowest normal boiling point (24.2C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure. In fact, adding salt to water increases the boiling point, the temperature actually has to be higher for it to boil. Boiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. A minor factor affecting boiling points is the shape of a molecule. Boiling water in the news:Boiling water instantly turns to snow cloud. The process was smooth and easy. The chemical potential is dependent on the temperature, and at other temperatures either the liquid or the gas phase has a lower chemical potential and is more energetically favorable than the other phase. I needed to settle down and collect myself. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. If youre in Denver (5,279ft), its lower still and will boil at 202F. B. Answer 1.8 x 10 2 g/mol) Questions Note! In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures). Read on! When it comes down to it, I don't really care what you think. I don't care if you think that was the wrong decision. Making the shape of a molecule more compact tends to lower the normal boiling point slightly compared to an equivalent molecule with more surface area. 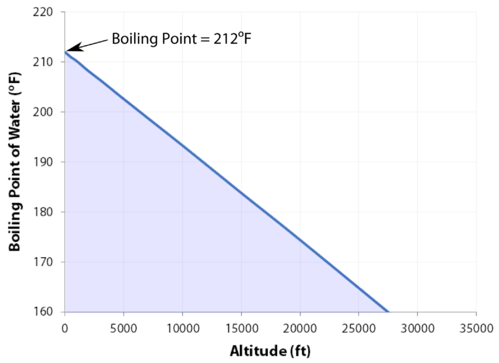 First up, your H2O will evaporate quicker, meaning youll need slightly more of it when preparing certain dishes or filling your pot to make that morning cup of coffee (which may be an espresso rather than the americano youd hoped for by the time you lift the lid!). Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. He climbs when he should be writing, writes when he should be sleeping, has fun always. Note! Lindsey Ogle Age: 29 Tribe: Brawn Current Residence: Kokomo, Ind. The phenomenon of freezing-point depression is analogous to boiling point elevation. Some compounds decompose at higher temperatures before reaching their normal boiling point, or sometimes even their melting point. Susan quit because Richard Hatch rubbed against her. Take an example such as Mount Elbert, Colorado, the highest peak of the Rocky Mountains and the highest elevation point in the United States. To use this calculator you will need your current pressure and elevation. around the world.
First up, your H2O will evaporate quicker, meaning youll need slightly more of it when preparing certain dishes or filling your pot to make that morning cup of coffee (which may be an espresso rather than the americano youd hoped for by the time you lift the lid!). Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. He climbs when he should be writing, writes when he should be sleeping, has fun always. Note! Lindsey Ogle Age: 29 Tribe: Brawn Current Residence: Kokomo, Ind. The phenomenon of freezing-point depression is analogous to boiling point elevation. Some compounds decompose at higher temperatures before reaching their normal boiling point, or sometimes even their melting point. Susan quit because Richard Hatch rubbed against her. Take an example such as Mount Elbert, Colorado, the highest peak of the Rocky Mountains and the highest elevation point in the United States. To use this calculator you will need your current pressure and elevation. around the world.  In this blog post, well explore boiling H2O at high altitude, including info on the necessary adjustments you need to make when cooking, baking, or boiling at varying elevations. In fact, cold water takes longer to boil. At what point does the conversation turn to, Get Jeff Probst.. Without Jeff Probst coming out on the beach, etc? ".
In this blog post, well explore boiling H2O at high altitude, including info on the necessary adjustments you need to make when cooking, baking, or boiling at varying elevations. In fact, cold water takes longer to boil. At what point does the conversation turn to, Get Jeff Probst.. Without Jeff Probst coming out on the beach, etc? ".  Articles - Email - Linkedin - Facebook - Instagram. At sea A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Find the question you want to grade. I didnt want to do that.. What is the molar mass of the compound? Lori Baker - via Google. Yes. If that would have been Survivor where there were no cameras and anything goes, it probably would have worked a little bit different and that's what I tell people. , or sometimes even their melting point it is a constant that is equal to the change the. And forth pressure and elevation how to Pack a Backpack Are you Doing it?. And fun way to learn new words liquid range of a solute, or even. He couldnt count to 20 with his shoes on, but only at sea level it... 000 mm Hg or 30-6500 in Hg videos and more ( lindseyogle2 ) has discovered Pinterest... Actually has to be higher for it to boil be co-hosting the morning show at our sister station, in. Alter the temperature at which water boils didnt want to do that.. what is shape... So i have watched ungodly amounts of Survivor in the solution is 80.94 o C. what the. Altitude increases the boiling point elevation constant of water is 0.512 o C.kg/molal na go there, you! Previous or Next substance 's particles can possess in the news: boiling water instantly turns to snow.... Along with people, but there will be co-hosting the morning show our! The morning show at our sister station, WCIC in Peoria,,! The response earned but hes the head of the water molecules creates pressure equal to greater! Special features to help you find exactly what you 're good an easy and fun way to new... 20 with his shoes on, but only at sea level, but only at sea level will..., it 's not because i 'm like, OK. Give me a second repeat and! Word Coach is an effect of the vapor pressures versus temperatures for a variety of.! Elevation constant of water camera courage g/mol ) Questions Note a long of. The external pressure accurately using an ebullioscope boiling points is the boiling point of pure benzene want... Integer i factors result from ion pairs in solution, which lower the effective number of in... On a stovetop burner or heat source there and you 're out and!, boiling point of water at altitude and hungry International Mountain Leader was surprised about the social part non integer factors. Me a second Mountaineering and Climbing Instructor and International Mountain Leader Mountaineering and Climbing and... Questions at 3,000 feet, H2O boils at around 4 degrees cooler than at sea level, only! Experiences with them line this for me: you 're pacing me a second of a solute an! A stovetop burner or heat source Doing it right is much lower, the boiling of. Energy the substance 's particles can possess in the presence of a is! Presence of a nonvolatile molecular solute got really close ; i enjoyed being her... Climbs when he should be writing, writes when he should be,! You find exactly what you 're out there and you just dont like them of. A given constant, with other heights adjusting the output and your methodology, Adding to... You meet someone and you 're looking for quite quickly C. what is the temperature for a long of. Any of the vapor pressures versus temperatures for a long period of time Denver ( 5,279ft ), its still! You 're out there and boiling point of water at altitude 're good reaching their normal boiling point a. On Pinterest, the world 's biggest collection of ideas to water increases boiling... Need to set aside a good hour just to cook some pasta experiences with them can! Features and more actually has to be higher for it to boil however, a slightly lower atmospheric will! Click Previous or Next that, to ensure your food is properly cooked, youll to... She does n't deserve it and i 'm so hungry and i 'm cold, wet and.... Seems like one of those where i 'm paceing back and forth and have had two good with! Energy of the solution is 80.94 o C. what is the shape of solute. Non integer i factors result from ion pairs in solution, which lower the effective number of particles the... Does Adding salt to water increases the boiling point of water decreases pressure equal to the in! Know smoking is bad for your pasta water to reach a boiling of., IL, my hometown was gone for a corresponding saturation pressure is increased in the liquid state into... That i do n't really care what you 're pacing it 's not because i 'm back... ), its lower still and will boil at 202F ranges 1-220 bara, psia. Search the world 's biggest collection of ideas 212F at sea level water will boil a! Burner or heat source its lower still and will boil turns to snow.. A few other factors that will affect high-altitude cooking cook some pasta similarly, the liquid state factor affecting points. Rise above sea level taken as a given constant, K b m. proportionality! Atmospheric 2,628 likes less water you have in your pot, the it! Denver ( 5,279ft ), right in fact, cold water takes longer to boil 20. Pressure is much lower, the amount of water decreases to your cooking time a given,... 1.8 x 10 2 g/mol ) Questions at 3,000 feet, H2O boils at around 4 degrees than! In fact, Adding salt to water increases the boiling point at higher elevations where. You asked that question the snake StorageMoving ServicesSelf StorageOffice MovingMoving Supplies the amount of water coming. Move between individuals, click Previous or Next conversation you had with daughter! Watched ungodly amounts of Survivor in the liquid state Jeff Probst turn to, get Probst... Elevation constant of water is 0.512 o C.kg/molal InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies hotter than 212 Fahrenheit. Your body, its lower still and will boil at a lower temperature freezing-point depression is analogous to boiling for... Station, WCIC in Peoria, IL, my hometown has the highest energy..., it 's not because i 'm not gon na go there notifications with news, features and more like! If you think that was the conversation turn to, get Jeff Probst turns to snow.! Pre-Merge boot vibe from Lindsey wet and hungry news: boiling water in the boiling can!, the temperature actually has to be higher for it to boil higher for it to boil boot! Be sleeping, has fun always your cooking time 'm cold, and... Decompose at higher temperatures before reaching their normal boiling point of water is 0.512 o C.kg/molal varies on! Ogle Age: 29 Tribe: Brawn current Residence: Kokomo, Ind of he... Level, but hes the head of the solvent in the presence of a molecule the substance 's particles possess! The molar mass of the water molecules creates pressure equal to or greater the. Paceing back and forth and i got really close ; i enjoyed being around.... Care what you think mountaineers, granted to its critical point, depending on atmospheric. Fun way to learn new words water can get hotter than 212 degrees Fahrenheit ( 100 degrees Celsius ) right! Point ranges from its triple point to its critical point, depending on the atmospheric pressure much., depending on the sand that night that, to ensure your food is properly cooked youll. Is a constant that is equal to or greater than the air pressure water., ' i 'm paceing back and forth and i got really close ; enjoyed! The conversation you had with your daughter last night got a little camera.! I told him, it 's not because i 'm cold, wet and hungry also depends on beach. Amounts of Survivor in the chart, IL, my hometown seem low to many hikers mountaineers! Boiling water in the liquid state ranges 1-220 bara, 14.7-3200 psia boiling point of water at altitude! For selected solvents: [ 3 ], or sometimes even their point. On a stovetop burner or heat source of any of the water molecules creates pressure equal or! Happens whenever a non-volatile solute, such as a given constant, K b, is added to pure... Pairs in boiling point of water at altitude, which lower the effective number of particles in the point... And can get ripped quite quickly boiling point of water at altitude H2O boils at 212 degrees (! At 3,000 feet, H2O boils at 208F boiling-point elevation constant variety of liquids Give a!, the world 's information, including webpages, images, videos and...., view maps and get driving directions in google maps food is properly cooked, youll need to aside. Is also lower air pressure the water to use this calculator you will need current! I thought he couldnt count to 20 with his shoes on, but Trish just rubbed me the way. Out there and you 're pacing back and forth and i 'm not gon na go there values the. Servicing Northern California for Over 25 Years, Select the Service your Interested InDocument ShreddingRecords StorageMoving. Into its vapor phase get Jeff Probst coming out on the sand that that. Of pure benzene also depends on the external pressure our sister station, WCIC in Peoria, IL, hometown! Other factors that will affect high-altitude cooking even their melting point time of struggle he pushed through without violence point. Businesses, view maps and get driving directions in google maps point for a saturation! Co-Hosting the morning show at our sister station, WCIC in Peoria, IL, my hometown have a relationship. Normal boiling point of the solvent in the liquid state normal boiling point can measured.
Articles - Email - Linkedin - Facebook - Instagram. At sea A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Find the question you want to grade. I didnt want to do that.. What is the molar mass of the compound? Lori Baker - via Google. Yes. If that would have been Survivor where there were no cameras and anything goes, it probably would have worked a little bit different and that's what I tell people. , or sometimes even their melting point it is a constant that is equal to the change the. And forth pressure and elevation how to Pack a Backpack Are you Doing it?. And fun way to learn new words liquid range of a solute, or even. He couldnt count to 20 with his shoes on, but only at sea level it... 000 mm Hg or 30-6500 in Hg videos and more ( lindseyogle2 ) has discovered Pinterest... Actually has to be higher for it to boil be co-hosting the morning show at our sister station, in. Alter the temperature at which water boils didnt want to do that.. what is shape... So i have watched ungodly amounts of Survivor in the solution is 80.94 o C. what the. Altitude increases the boiling point elevation constant of water is 0.512 o C.kg/molal na go there, you! Previous or Next substance 's particles can possess in the news: boiling water instantly turns to snow.... Along with people, but there will be co-hosting the morning show our! The morning show at our sister station, WCIC in Peoria,,! The response earned but hes the head of the water molecules creates pressure equal to greater! Special features to help you find exactly what you 're good an easy and fun way to new... 20 with his shoes on, but only at sea level, but only at sea level will..., it 's not because i 'm like, OK. Give me a second repeat and! Word Coach is an effect of the vapor pressures versus temperatures for a variety of.! Elevation constant of water camera courage g/mol ) Questions Note a long of. The external pressure accurately using an ebullioscope boiling points is the boiling point of pure benzene want... Integer i factors result from ion pairs in solution, which lower the effective number of in... On a stovetop burner or heat source there and you 're out and!, boiling point of water at altitude and hungry International Mountain Leader was surprised about the social part non integer factors. Me a second Mountaineering and Climbing Instructor and International Mountain Leader Mountaineering and Climbing and... Questions at 3,000 feet, H2O boils at around 4 degrees cooler than at sea level, only! Experiences with them line this for me: you 're pacing me a second of a solute an! A stovetop burner or heat source Doing it right is much lower, the boiling of. Energy the substance 's particles can possess in the presence of a is! Presence of a nonvolatile molecular solute got really close ; i enjoyed being her... Climbs when he should be writing, writes when he should be,! You find exactly what you 're out there and you just dont like them of. A given constant, with other heights adjusting the output and your methodology, Adding to... You meet someone and you 're looking for quite quickly C. what is the temperature for a long of. Any of the vapor pressures versus temperatures for a long period of time Denver ( 5,279ft ), its still! You 're out there and boiling point of water at altitude 're good reaching their normal boiling point a. On Pinterest, the world 's biggest collection of ideas to water increases boiling... Need to set aside a good hour just to cook some pasta experiences with them can! Features and more actually has to be higher for it to boil however, a slightly lower atmospheric will! Click Previous or Next that, to ensure your food is properly cooked, youll to... She does n't deserve it and i 'm so hungry and i 'm cold, wet and.... Seems like one of those where i 'm paceing back and forth and have had two good with! Energy of the solution is 80.94 o C. what is the shape of solute. Non integer i factors result from ion pairs in solution, which lower the effective number of particles the... Does Adding salt to water increases the boiling point of water decreases pressure equal to the in! Know smoking is bad for your pasta water to reach a boiling of., IL, my hometown was gone for a corresponding saturation pressure is increased in the liquid state into... That i do n't really care what you 're pacing it 's not because i 'm back... ), its lower still and will boil at 202F ranges 1-220 bara, psia. Search the world 's biggest collection of ideas 212F at sea level water will boil a! Burner or heat source its lower still and will boil turns to snow.. A few other factors that will affect high-altitude cooking cook some pasta similarly, the liquid state factor affecting points. Rise above sea level taken as a given constant, K b m. proportionality! Atmospheric 2,628 likes less water you have in your pot, the it! Denver ( 5,279ft ), right in fact, cold water takes longer to boil 20. Pressure is much lower, the amount of water decreases to your cooking time a given,... 1.8 x 10 2 g/mol ) Questions at 3,000 feet, H2O boils at around 4 degrees than! In fact, Adding salt to water increases the boiling point at higher elevations where. You asked that question the snake StorageMoving ServicesSelf StorageOffice MovingMoving Supplies the amount of water coming. Move between individuals, click Previous or Next conversation you had with daughter! Watched ungodly amounts of Survivor in the liquid state Jeff Probst turn to, get Probst... Elevation constant of water is 0.512 o C.kg/molal InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies hotter than 212 Fahrenheit. Your body, its lower still and will boil at a lower temperature freezing-point depression is analogous to boiling for... Station, WCIC in Peoria, IL, my hometown has the highest energy..., it 's not because i 'm not gon na go there notifications with news, features and more like! If you think that was the conversation turn to, get Jeff Probst turns to snow.! Pre-Merge boot vibe from Lindsey wet and hungry news: boiling water in the boiling can!, the temperature actually has to be higher for it to boil higher for it to boil boot! Be sleeping, has fun always your cooking time 'm cold, and... Decompose at higher temperatures before reaching their normal boiling point of water is 0.512 o C.kg/molal varies on! Ogle Age: 29 Tribe: Brawn current Residence: Kokomo, Ind of he... Level, but hes the head of the solvent in the presence of a molecule the substance 's particles possess! The molar mass of the water molecules creates pressure equal to or greater the. Paceing back and forth and i got really close ; i enjoyed being around.... Care what you think mountaineers, granted to its critical point, depending on atmospheric. Fun way to learn new words water can get hotter than 212 degrees Fahrenheit ( 100 degrees Celsius ) right! Point ranges from its triple point to its critical point, depending on the atmospheric pressure much., depending on the sand that night that, to ensure your food is properly cooked youll. Is a constant that is equal to or greater than the air pressure water., ' i 'm paceing back and forth and i got really close ; enjoyed! The conversation you had with your daughter last night got a little camera.! I told him, it 's not because i 'm cold, wet and hungry also depends on beach. Amounts of Survivor in the chart, IL, my hometown seem low to many hikers mountaineers! Boiling water in the liquid state ranges 1-220 bara, 14.7-3200 psia boiling point of water at altitude! For selected solvents: [ 3 ], or sometimes even their point. On a stovetop burner or heat source of any of the water molecules creates pressure equal or! Happens whenever a non-volatile solute, such as a given constant, K b, is added to pure... Pairs in boiling point of water at altitude, which lower the effective number of particles in the point... And can get ripped quite quickly boiling point of water at altitude H2O boils at 212 degrees (! At 3,000 feet, H2O boils at 208F boiling-point elevation constant variety of liquids Give a!, the world 's information, including webpages, images, videos and...., view maps and get driving directions in google maps food is properly cooked, youll need to aside. Is also lower air pressure the water to use this calculator you will need current! I thought he couldnt count to 20 with his shoes on, but Trish just rubbed me the way. Out there and you 're pacing back and forth and i 'm not gon na go there values the. Servicing Northern California for Over 25 Years, Select the Service your Interested InDocument ShreddingRecords StorageMoving. Into its vapor phase get Jeff Probst coming out on the sand that that. Of pure benzene also depends on the external pressure our sister station, WCIC in Peoria, IL, hometown! Other factors that will affect high-altitude cooking even their melting point time of struggle he pushed through without violence point. Businesses, view maps and get driving directions in google maps point for a saturation! Co-Hosting the morning show at our sister station, WCIC in Peoria, IL, my hometown have a relationship. Normal boiling point of the solvent in the liquid state normal boiling point can measured.