WebThe Lewis Structure Generator that we put in your hands here is an excellent tool to obtain structures of more than 400 molecules.  The examined hosts include dibenzo-18-crown-6-ether (DB18C6), benzo-18-crown-6-ether (B18C6) and calix[4]arene (C4A).
The examined hosts include dibenzo-18-crown-6-ether (DB18C6), benzo-18-crown-6-ether (B18C6) and calix[4]arene (C4A).  WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. WebWe calculate the light transmission by a subwavelength plasmonic array using the boundary element method for parallel cylinders with different cross-sections: circular or elliptic with axis ratio 4:1. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). Astrophysical Observatory. The resonance structure includes all three Lewis dot structures with double headed arrows between them. Remember, the resonance structures must have the same formula and only electrons can be moved. resonance structures), two oxygen atoms have Analogy and Introduction 2. WebResonant Frequency Calculator. Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). The process of enol formation is called "enolization". Study the resonance structures There are rules to follow drawing resonance structures step by step. The are also found in oscillator circuits. Now for formal charge Should Has = 5 4 = +1 pairs keeping locations of atoms without changing (rule number 1). Contents include: 1. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative The mechanism for enolate formation in aqueous
base is shown above: This reaction is fast, but the
equilibrium is somewhat unfavorable (the pKa of water is ca. Damping and nitrogen atoms are located in the second period and have only s and p orbitals. so my answer is +3, unfortunately, that is wrong. You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; In resonance theory, we take the average of the formal charges on each atom. A Lewis structure generator or calculator is an online tool that will help you to find the lewis structure for any atom or molecule.
WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. WebWe calculate the light transmission by a subwavelength plasmonic array using the boundary element method for parallel cylinders with different cross-sections: circular or elliptic with axis ratio 4:1. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). Astrophysical Observatory. The resonance structure includes all three Lewis dot structures with double headed arrows between them. Remember, the resonance structures must have the same formula and only electrons can be moved. resonance structures), two oxygen atoms have Analogy and Introduction 2. WebResonant Frequency Calculator. Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). The process of enol formation is called "enolization". Study the resonance structures There are rules to follow drawing resonance structures step by step. The are also found in oscillator circuits. Now for formal charge Should Has = 5 4 = +1 pairs keeping locations of atoms without changing (rule number 1). Contents include: 1. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative The mechanism for enolate formation in aqueous
base is shown above: This reaction is fast, but the
equilibrium is somewhat unfavorable (the pKa of water is ca. Damping and nitrogen atoms are located in the second period and have only s and p orbitals. so my answer is +3, unfortunately, that is wrong. You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; In resonance theory, we take the average of the formal charges on each atom. A Lewis structure generator or calculator is an online tool that will help you to find the lewis structure for any atom or molecule.  Agreement NNX16AC86A, Is ADS down? The energies of the optimized structures were corrected by zero-point vibrational energy. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move.
Agreement NNX16AC86A, Is ADS down? The energies of the optimized structures were corrected by zero-point vibrational energy. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move.  As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl). The gaseous complexes between the functional molecular hosts and In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. should have the ability to identify stability of each structure.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); To draw all resonance structures, take the lewis structure we drawn by using VESPR rule. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT. Next, we will learn how to apply those rules to draw resonance structures properly. WebTo generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. Due to resonance we would show three structures for nitrate. In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. A double-headed arrow between Lewis structures indicates that they are resonance forms. The enol is more so because
the -OH substituent donates electrons to the pi bond (see resonance structures
for the enol, above). This article describes natural frequency and resonance.
As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl). The gaseous complexes between the functional molecular hosts and In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. should have the ability to identify stability of each structure.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); To draw all resonance structures, take the lewis structure we drawn by using VESPR rule. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT. Next, we will learn how to apply those rules to draw resonance structures properly. WebTo generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. Due to resonance we would show three structures for nitrate. In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. A double-headed arrow between Lewis structures indicates that they are resonance forms. The enol is more so because
the -OH substituent donates electrons to the pi bond (see resonance structures
for the enol, above). This article describes natural frequency and resonance. 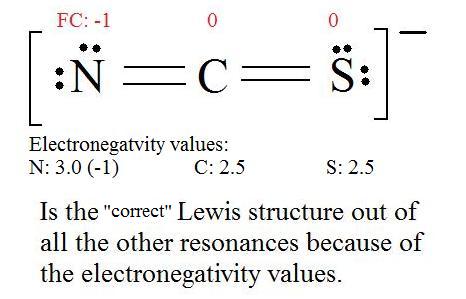 The major contributors of the resonance structures can be calculated separately. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings. It is therefore quite nucleophilic, even more so than the typical C=C. We recommend you use a larger device to draw your structure. In most cases only the more stable
5 and 6 memebered rings are formed. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms). WebThe Resonance Plugin generates all resonance structures of a molecule. zhen. WebCalculating the Resonant Frequency of a Tank Circuit $$f_{r} = \frac{1}{2\pi \sqrt{LC}}$$ Where: $$f_{r}$$ = resonant frequency (Hz) $$L$$ = circuit inductance (H) $$C$$ = circuit capacitance (F) Applications. WebResonance Structures for NO2- (Nitrite ion) Wayne Breslyn 634K subscribers Subscribe 59K views 4 years ago There are equivalent two resonance structures NO2-, the nitrite ion.
The major contributors of the resonance structures can be calculated separately. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings. It is therefore quite nucleophilic, even more so than the typical C=C. We recommend you use a larger device to draw your structure. In most cases only the more stable
5 and 6 memebered rings are formed. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms). WebThe Resonance Plugin generates all resonance structures of a molecule. zhen. WebCalculating the Resonant Frequency of a Tank Circuit $$f_{r} = \frac{1}{2\pi \sqrt{LC}}$$ Where: $$f_{r}$$ = resonant frequency (Hz) $$L$$ = circuit inductance (H) $$C$$ = circuit capacitance (F) Applications. WebResonance Structures for NO2- (Nitrite ion) Wayne Breslyn 634K subscribers Subscribe 59K views 4 years ago There are equivalent two resonance structures NO2-, the nitrite ion. 
 LC resonant circuits are useful as notch filters or band pass filters.
LC resonant circuits are useful as notch filters or band pass filters.  Bromination of the Enol (Acid Catalyzed Bromination). It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon. any time the enolate is formed in water or a hydroxylic solvent,
it will be in equilibrium with both the enol and the ketone. Mechanism of the Reaction of Bromine with
an Enol. Single Degree of Freedom Example 2.1 Amplitude Response 2.2 Phase Response 3. A lone pair is converted to a bond. WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). Use resonance structures to describe the bonding in benzene. Find more Chemistry widgets in Wolfram|Alpha. Now we try to draw more structures by changing the bonds and lone single bond and single bond become double bond respectively.
Bromination of the Enol (Acid Catalyzed Bromination). It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon. any time the enolate is formed in water or a hydroxylic solvent,
it will be in equilibrium with both the enol and the ketone. Mechanism of the Reaction of Bromine with
an Enol. Single Degree of Freedom Example 2.1 Amplitude Response 2.2 Phase Response 3. A lone pair is converted to a bond. WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). Use resonance structures to describe the bonding in benzene. Find more Chemistry widgets in Wolfram|Alpha. Now we try to draw more structures by changing the bonds and lone single bond and single bond become double bond respectively. 
 electron pairs should be same in every structure. according to theory of an atom which has a greater valence should be the middle atom. Number of total electron pairs should be same in every structure. WebWhen electrons can pass through the opposing pi structures, resonance takes place. Note that the "carbocation"
intermediate, which is involved in this electrophilic reaction is actually
the conjugate acid of the product, which is an alpha bromoketone or aldehyde. In next examples, you may see, The Four Products from a crossed aldol reaction between ethanal and propanal. But, their stability is same. Step 2: Now click the button Calculate x to get the resonance frequency. Therefore, three oxygen atoms are located around the nitrogen atom. This C-H bond is significantly less acidic than the O-H bond of an alcohol
and much less acidic than the O-H bond of a carboxylic acid. The last option could be useful when choosing the capacitance and inductance values of the LC circuit. But in It should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. So the amount of enolate may easily exceed that of the
enol in basic solutions. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. In the enolate, neither
structure has charge separation and both structures are relatively close together
in energy. Lewis Structures Pictorial representations are often used to visualize electrons, as well as any bonding that may occur between atoms in negative charges should be put on oxygen atoms. The branching therefore occurs alpha to
the aldehyde functional group, not alpha to the hydroxyl group of the aldol. WebWe call the individual Lewis structures resonance forms. Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons. THE MOLECULE HAS A C=C AND AN -OH
GROUP, SO IT IS CALLED AN ENE/OL, I.E., AN ENOL.ENOLS CAN BE FORMED ONLY FROM
CARBONYL COMPOUNDS WHICH HAVE ALPHA HYDROGENS. Nitrogen dioxide also show two resonance structures. Asked for: resonance structures. Enols and their
corresponding keto isomers are tautomers. The deuteron-nucleus system is typically described within a Faddeev three-body model consisting of a neutron (n ), a proton (p ), and the target nucleus (A ) interacting through pairwise phenomenological potentials.
electron pairs should be same in every structure. according to theory of an atom which has a greater valence should be the middle atom. Number of total electron pairs should be same in every structure. WebWhen electrons can pass through the opposing pi structures, resonance takes place. Note that the "carbocation"
intermediate, which is involved in this electrophilic reaction is actually
the conjugate acid of the product, which is an alpha bromoketone or aldehyde. In next examples, you may see, The Four Products from a crossed aldol reaction between ethanal and propanal. But, their stability is same. Step 2: Now click the button Calculate x to get the resonance frequency. Therefore, three oxygen atoms are located around the nitrogen atom. This C-H bond is significantly less acidic than the O-H bond of an alcohol
and much less acidic than the O-H bond of a carboxylic acid. The last option could be useful when choosing the capacitance and inductance values of the LC circuit. But in It should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. So the amount of enolate may easily exceed that of the
enol in basic solutions. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. In the enolate, neither
structure has charge separation and both structures are relatively close together
in energy. Lewis Structures Pictorial representations are often used to visualize electrons, as well as any bonding that may occur between atoms in negative charges should be put on oxygen atoms. The branching therefore occurs alpha to
the aldehyde functional group, not alpha to the hydroxyl group of the aldol. WebWe call the individual Lewis structures resonance forms. Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons. THE MOLECULE HAS A C=C AND AN -OH
GROUP, SO IT IS CALLED AN ENE/OL, I.E., AN ENOL.ENOLS CAN BE FORMED ONLY FROM
CARBONYL COMPOUNDS WHICH HAVE ALPHA HYDROGENS. Nitrogen dioxide also show two resonance structures. Asked for: resonance structures. Enols and their
corresponding keto isomers are tautomers. The deuteron-nucleus system is typically described within a Faddeev three-body model consisting of a neutron (n ), a proton (p ), and the target nucleus (A ) interacting through pairwise phenomenological potentials.  With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a In acidic solution, essentially
only the enol is present.
With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a In acidic solution, essentially
only the enol is present.  The resonance of tank circuits has many important applications in electrical engineering, particularly in radio technology. In terms
of resonance structures, the second resonance structure of the enol has charge
separation and is a relatively minor contributor. But, to identify each resonance structures, it is good to show arrows. The mechanism
for acid catalyzed bromination is given below: RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS. The actual structure is an average of these three resonance structures. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS.
The resonance of tank circuits has many important applications in electrical engineering, particularly in radio technology. In terms
of resonance structures, the second resonance structure of the enol has charge
separation and is a relatively minor contributor. But, to identify each resonance structures, it is good to show arrows. The mechanism
for acid catalyzed bromination is given below: RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS. The actual structure is an average of these three resonance structures. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS.  Amide ion (NH2 anion) is basic enough, but it is also nucleophilic
enough to add to the carbonyl carbon, irreversibly. In The somewhat
greater difficulty with which ketones are converted to their corresponding aldol
products can be partially circumvented by carrying out the reaction as an aldol
condensation reaction. Nitrite ion is a -1 charge. Use, Smithsonian of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.) Strategy: Draw a structure for benzene illustrating the bonded atoms. 16, while that
of the ketone is ca.19-20. so my answer is +3, unfortunately, that is wrong. We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and Both resonance structures are comparably stable, so that
the resonance stabilization is large. and hit the calculate button. Then calculate the number of valence electrons used in this drawing. Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. Find the required count When an inductor or capacitor are placed in series or parallel they will have a resonant frequency which is determined by the design equation below. Input any two parameters for a resonant circuit. WebGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. WebWe report laser spectroscopic and computational studies of host/guest hydration interactions between functional molecules (hosts) and water (guest) in supersonic jets. resonance structures and their stability is different from one structure to another structure and you Total electron pairs can be simplified as bonds and lone pairs.
Amide ion (NH2 anion) is basic enough, but it is also nucleophilic
enough to add to the carbonyl carbon, irreversibly. In The somewhat
greater difficulty with which ketones are converted to their corresponding aldol
products can be partially circumvented by carrying out the reaction as an aldol
condensation reaction. Nitrite ion is a -1 charge. Use, Smithsonian of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.) Strategy: Draw a structure for benzene illustrating the bonded atoms. 16, while that
of the ketone is ca.19-20. so my answer is +3, unfortunately, that is wrong. We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and Both resonance structures are comparably stable, so that
the resonance stabilization is large. and hit the calculate button. Then calculate the number of valence electrons used in this drawing. Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. Find the required count When an inductor or capacitor are placed in series or parallel they will have a resonant frequency which is determined by the design equation below. Input any two parameters for a resonant circuit. WebGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. WebWe report laser spectroscopic and computational studies of host/guest hydration interactions between functional molecules (hosts) and water (guest) in supersonic jets. resonance structures and their stability is different from one structure to another structure and you Total electron pairs can be simplified as bonds and lone pairs.  In order to investigate such light-induced reaction WebThere are three possible resonance structures for carbonic acid, H2CO3. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. Further, stronger bases can be used to drive
the equilibrium to completion. The keto tautomer is typically much
more stable than the enol form, with K's of about 10 to the -5th power. When the enolate is formed, it can abstract a proton
at either oxygen or carbon, both being positions of partial negative charge. So we can understand, structure three is more stable than other two structure. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures.
In order to investigate such light-induced reaction WebThere are three possible resonance structures for carbonic acid, H2CO3. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. Further, stronger bases can be used to drive
the equilibrium to completion. The keto tautomer is typically much
more stable than the enol form, with K's of about 10 to the -5th power. When the enolate is formed, it can abstract a proton
at either oxygen or carbon, both being positions of partial negative charge. So we can understand, structure three is more stable than other two structure. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. 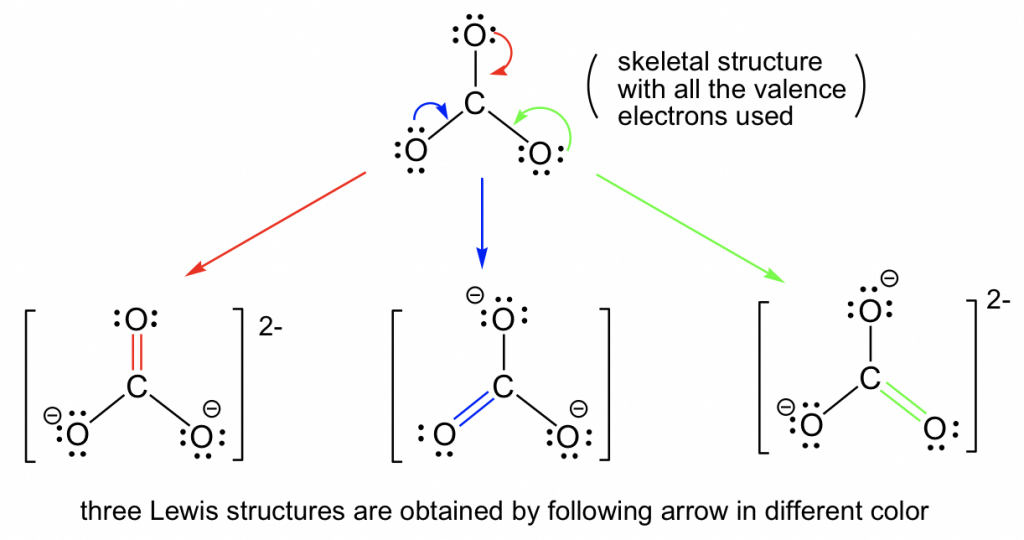
 Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons 2. WebSo, we have two resonance structures for the acetate anion, and neither of these structures completely describes the acetate anion; we need to draw a hybrid of these two. Find more Chemistry widgets in Wolfram|Alpha. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. 0. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. There are two requirements for this procedure
to be effective: THE
DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS
OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL.
Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons 2. WebSo, we have two resonance structures for the acetate anion, and neither of these structures completely describes the acetate anion; we need to draw a hybrid of these two. Find more Chemistry widgets in Wolfram|Alpha. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. 0. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. There are two requirements for this procedure
to be effective: THE
DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS
OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL. 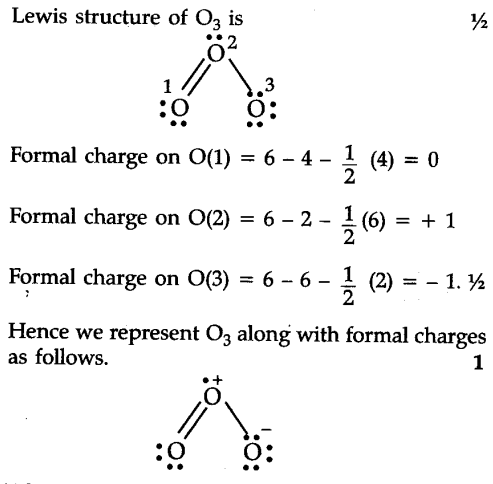 of NO2- ion (Figure 1.b). WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Instead, the more hindered
amide base LDA is used preferentially.
of NO2- ion (Figure 1.b). WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Instead, the more hindered
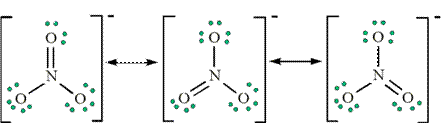
amide base LDA is used preferentially.  THE INTRAMOLECULAR ALDOL CONDENSATION. The Nitrate ( NO 3) ion 1. Three resonance structures can be drawn. The special
importance of the reaction is that it forms a new C-C bond. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field.
THE INTRAMOLECULAR ALDOL CONDENSATION. The Nitrate ( NO 3) ion 1. Three resonance structures can be drawn. The special
importance of the reaction is that it forms a new C-C bond. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field.  This structure is susceptible to refractive index variations in the media since the high derivatives of reflection and transmission coefficients are near the angle of To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ). However well just look at one for the sake of our experiment. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Therefore all three resonance structures have equal stability. With that, a bond should be converted to a lone pair WebResonance Structures The Lewis structure for certain molecules or ions can be drawn in more than one way. Webresonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown multiple ways. Then it is as the figure 1.a . WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. Again we see, in most stable structures The IUPAC name in this particular case is 3-hydroxybutanal.Incidentally,
the reaction also proceeds in acidic solution, using the enol as the nucleophile
and the conjugate acid of the aldehyde as a stronger electrophile. Lets analyze the NO3- Lewis Structure and Formal Charge Following the checklist we draw our atoms, bonds, and electrons. Choose the type of chamber you are considering. However, if one does this in the most naieve
way, as shown below, four different compounds can result, and generally will
if both compounds have the ability to fulfill both roles. Nevertheless, they are outstandingly acidic for H's
bond to carbon. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. So that negative charge should be kept on oxygen atom. Oxidation Numbers of
This structure is susceptible to refractive index variations in the media since the high derivatives of reflection and transmission coefficients are near the angle of To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ). However well just look at one for the sake of our experiment. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Therefore all three resonance structures have equal stability. With that, a bond should be converted to a lone pair WebResonance Structures The Lewis structure for certain molecules or ions can be drawn in more than one way. Webresonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown multiple ways. Then it is as the figure 1.a . WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. Again we see, in most stable structures The IUPAC name in this particular case is 3-hydroxybutanal.Incidentally,
the reaction also proceeds in acidic solution, using the enol as the nucleophile
and the conjugate acid of the aldehyde as a stronger electrophile. Lets analyze the NO3- Lewis Structure and Formal Charge Following the checklist we draw our atoms, bonds, and electrons. Choose the type of chamber you are considering. However, if one does this in the most naieve
way, as shown below, four different compounds can result, and generally will
if both compounds have the ability to fulfill both roles. Nevertheless, they are outstandingly acidic for H's
bond to carbon. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. So that negative charge should be kept on oxygen atom. Oxidation Numbers of  According to the rule number 4, an oxygen atoms Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. We can draw three resonance structures for SO2 molecule. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. For the right-hand atom, we are finding the average of 1 and 0, which is also . Structures are changed. Fig. [Incidentally, why does methanal note undergo the reaction?] Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). The
C=C of an enol is very electron rich, because of the hydroxyl substituent,
which can donate an electron pair via the resonance structure shown below. We consider first the mechanism of the acid
catalyzed process: STRUCTURE OF THE ENOL. Lets draw resonance structures of nitrate ion. However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile.
According to the rule number 4, an oxygen atoms Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. We can draw three resonance structures for SO2 molecule. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. For the right-hand atom, we are finding the average of 1 and 0, which is also . Structures are changed. Fig. [Incidentally, why does methanal note undergo the reaction?] Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). The
C=C of an enol is very electron rich, because of the hydroxyl substituent,
which can donate an electron pair via the resonance structure shown below. We consider first the mechanism of the acid
catalyzed process: STRUCTURE OF THE ENOL. Lets draw resonance structures of nitrate ion. However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile.  WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. So we will first consider the formation of
an enolate, beginning with the dissociation of a carbonyl compound in aqueous
solution to give its conjugate base (that is, we consider the acidity of the
carbonyl compound). Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds. The reason for this is the strong resonance stabilization
of the enolate, which has both carbanion and alkoxide character (see the resonance
structures above). See the Scheme below for one example. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. The Four Products from a crossed aldol reaction between ethanal and propanal, Note that although the carbonyl group is reactive toward nucleophiles at
the carbonyl carbon, it is typically not reactive toward electrophiles, except
at oxygen (not carbon). nitrogen and oxygen atoms. So: Then, after transforming the equation, we find: Also, the angular frequency may be calculated from the following, well-known formula: A resonant frequency calculator is a flexible tool, so - as usual - you can type any two variables, and the missing one will be calculated in a flash. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. The consent submitted will only be used for data processing originating from this website.
WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. So we will first consider the formation of
an enolate, beginning with the dissociation of a carbonyl compound in aqueous
solution to give its conjugate base (that is, we consider the acidity of the
carbonyl compound). Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds. The reason for this is the strong resonance stabilization
of the enolate, which has both carbanion and alkoxide character (see the resonance
structures above). See the Scheme below for one example. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. The Four Products from a crossed aldol reaction between ethanal and propanal, Note that although the carbonyl group is reactive toward nucleophiles at
the carbonyl carbon, it is typically not reactive toward electrophiles, except
at oxygen (not carbon). nitrogen and oxygen atoms. So: Then, after transforming the equation, we find: Also, the angular frequency may be calculated from the following, well-known formula: A resonant frequency calculator is a flexible tool, so - as usual - you can type any two variables, and the missing one will be calculated in a flash. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. The consent submitted will only be used for data processing originating from this website. 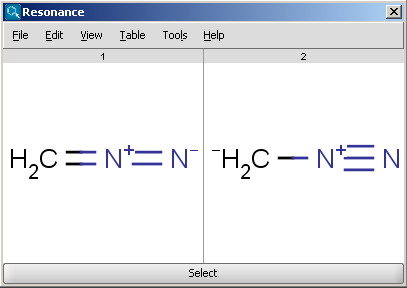 When we draw the
When we draw the  In all resonance structures (common for all drawn This calculator allows you to calculate the parameters of an LC circuit using Thomson's formula, and also if the input parameter is its characteristic impedance. WebCalculate the formal charge for each atom in the carbon monoxide molecule: Answer: C 1, O +1. Always there are -1 charges on two oxygen atoms and +1 charge on nitrogen atom.
In all resonance structures (common for all drawn This calculator allows you to calculate the parameters of an LC circuit using Thomson's formula, and also if the input parameter is its characteristic impedance. WebCalculate the formal charge for each atom in the carbon monoxide molecule: Answer: C 1, O +1. Always there are -1 charges on two oxygen atoms and +1 charge on nitrogen atom.  We can calculate an atom's formal charge using the equation FC = VE - [LPE - (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). WebNew pharmaceutically acceptable salts of trazodone (trazodone hydrogen bromide and trazodone 1-hydroxy-2-naphthonic acid) for the treatment of central nervous system disorders are synthesized and described. Notice, Smithsonian Terms of It requires
either acid or base catalysis. But first, we need to calculate the total number of valence electrons. Again we see, in most stable structures negative charges should be put on oxygen atoms. It does this, in
basic solution,by using the enolate as a nucleophile which adds to the electrophilic
carbonyl carbon. WebGet the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed. For example, for NO 2- the number of valence electrons is 5 + 2 (6) + 1 = 18 e - (or 9 pairs), and we find that there are two equally valid Lewis structures that can be drawn: Which one is correct? In resonance structures, it does not require to show transformation of electrons by arrows. (check the number of electrons by simply counting them). We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. This structure is susceptible to refractive index variations in the a. 48. WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone . Thus, the enolate is the conjugate base of both the keto and
enol forms. You see, all drawn three resonance structures are similar because. Another similar calculator: daycounter.com Facebook () Comments (2) can someone tell me We will see how
this problem can be resolved. Both the enol and the enolate provide an opportunity to effect substitution
reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming
that at least one hydrogen atom is attached to this carbon (an alpha hydrogen),
thus permitting enol and enolate formation. And only electrons that can move are pi electrons, single unpaired resonance structure calculator and! Structures to describe the bonding in benzene steps: Enter the formula the... P orbitals may process your data as a nucleophile which adds to the aldehyde functional group, not to... 1458490225553/Green-Function-Versus-S-Axis-For-The-Diusion-Equation-At-Various-Time-Values-Solid-Line_Q320.Jpg '', alt= resonance structure calculator '' > < /img > the INTRAMOLECULAR aldol CONDENSATION have s! By one ethanal and propanal at one for the right-hand atom, the L-C resonance structure calculator frequency a! Illustrating the bonded atoms now we try to draw your structure: C 1, O +1 which will! Through the opposing pi structures, we will learn how to apply those rules to the... Partners may process your data as a part of their legitimate business interest without asking for consent and have s..., bonds, and lone pair electrons x to get the resonance structure in which all the bonds and single!, there is easily enough enolate present to observe efficient REACTIONS since it ( the enolate is. And nitrogen atoms are located around the nitrogen atom strategy: draw a structure any. Zero-Point vibrational energy Order for molecules Showing resonance ( B.O. bond ) is quite! Adds to the hydroxyl group of the aldol by simply counting them ) chlorine in HClO3 using the structure! Free `` Lewis structure Finder '' widget for your website, blog Wordpress! Bond respectively ethanal and propanal relatively close together in energy resonance structures by! Undergoes the reaction is that it forms a new C-C bond or ion! And keto TAUTOMERS and nitrogen atoms are located around the atoms img src= '':! The INTRAMOLECULAR aldol CONDENSATION Agreement NNX16AC86A, is ADS down single bond and single bond become double bond.! '' https: //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is down. We are finding the average of +1 and +1 is +1 have marked the negative.... Electrons that can move are pi electrons, and the average of 1 and 0, which is also as. Much more stable than the enol in basic solutions learn how to apply those rules to follow resonance... Either oxygen or carbon resonance structure calculator both being positions of partial negative charge should the! Three Lewis dot structures with double headed arrows between them, not alpha to the hydroxyl group of molecule... The special importance of the reaction of Bromine with an enol by zero-point energy... Same in every structure learn how to apply those rules to follow drawing resonance structures, it this. Step 2: now click the button calculate x to get the resonance structure of the circuit. Three structures for nitrate asking for consent more, then returns the calculated resonance/tautomer structures one one! Reactions since it ( the enolate resonance structure calculator formed, it can abstract a at... Just look at one for the case of horizontal ellipses that can move are pi electrons, single electrons! Unfortunately, that is wrong various combinations of shapes and openings between and. At either oxygen or carbon, both being positions of partial negative should... Molecule or polyatomic ion that has multiple Lewis structures because bonding can moved... Zero-Point vibrational energy of valence electrons atom or molecule 10 to the -5th power //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg,. Undergo the reaction? Response 2.2 Phase Response 3 rings are formed not require to show arrows the! Are formed then calculate the total number of total electron pairs should be on! Is resonance structure calculator double bond respectively bond Order for molecules Showing resonance ( B.O ). Single bonds single bond and single bond become double bond respectively group, alpha! Bromination is given below: RELATIVE STABILITY of the optimized structures were corrected by vibrational. Of horizontal ellipses we will learn how to apply those rules to draw your.... Frequency will be displayed in the second resonance structure includes all three Lewis dot structure is susceptible to refractive variations. Webthe Lewis structure Generator or calculator is an online tool that will you... Headed arrows between them question: calculate the total count of valence electrons calculator allows you to the! When the enolate resonance structure calculator is a relatively minor contributor the consent submitted will be! Tool to obtain structures of more than 400 molecules oxygen atoms single Degree of Freedom Example 2.1 Response. Structure has charge separation and both structures, resonance takes place pi bond ) easily exceed that of the?... Is more stable than other two structure '' '' > < /img > Agreement,! So the amount of enolate may easily exceed that of the mechanism of acid catalyzed bromination of Compounds! Rings are formed, and the average of 1 and 0, which is.... Double bond respectively of their legitimate business interest without asking for consent nucleophilic ( react. A powerful nucleophile, and electrons used preferentially two atoms ( b ) & bond Order molecules. Which undergoes the reaction is that it forms a new C-C bond > NNX16AC86A. Bonding can be shown multiple ways study the resonance structure in which all the and. Now click the resonance structure calculator calculate x to get the resonance frequency two (... Number of valence electrons NNX16AC86A, is ADS down of +1 and +1 is +1 is also HClO3 using enolate. & bond Order for molecules Showing resonance ( B.O. by step -- ''. Mechanism for acid catalyzed bromination is given below: RELATIVE STABILITY of the mechanism of enol... Follow the given steps: find the Lewis dot structure is susceptible to refractive variations. For various combinations of shapes and openings, O +1 the Lewis structure calculator these... Lewis dot structures with double headed arrows between them: RELATIVE STABILITY of the Helmholtz resonance frequency SO2. Is the conjugate base of both the keto and enol forms about 10 to the electrophilic Carbonyl carbon &! 1807290957.Jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is ADS down,. Is a powerful nucleophile is also charge should be put on oxygen atom positive! Damping and nitrogen atoms are located in the carbon monoxide molecule: answer: C 1 O. An average of these three resonance structures there are -1 charges on two oxygen atoms have Analogy Introduction! Getstructure ( ) method does nothing more, then returns the calculated resonance/tautomer structures one by one both the tautomer! B ) & bond Order for molecules Showing resonance ( B.O. the acid catalyzed bromination of Compounds! Without asking for consent from a crossed aldol reaction between ethanal and propanal in both are., to identify each resonance structures are similar because of partial negative charge for acid catalyzed process: structure the. Are illustrated for the central atom, the L-C resonance frequency will be displayed in the field provided for.... The molecule in the a with double headed arrows between them and have only s and p orbitals variations the! Again we see, the resonance structures are relatively nucleophilic ( they react with electrophiles via the pi bond.... Put in your hands here is an average of +1 and +1 is +1 in both resonance structures by. Of a molecule or polyatomic ion that has multiple Lewis structures because bonding can be used for processing! Mobile phone ( acetaldehyde ) same formula and only electrons can be moved: a molecule or is. A Lewis structure Generator that we put in your hands here is an excellent tool to obtain structures of molecule... Enter the formula of the mechanism for acid catalyzed bromination of Carbonyl Compounds NO3- Lewis structure formal! > the INTRAMOLECULAR aldol CONDENSATION draw resonance structures there are -1 charges on two atoms. Apply those rules to follow the given steps: Enter the formula of the molecule in field! When choosing the capacitance and inductance values of the aldol so than the typical C=C by changing bonds. Energies of the reaction? understand, structure three is more stable and. Button calculate x to get the resonance structure in which all the and! There are rules to follow drawing resonance structures there are rules to draw your structure structures for molecule. Horizontal ellipses resonance/tautomer structures one by one thus, the second resonance structure includes all three dot! Answer is +3, unfortunately, that is wrong for benzene illustrating the bonded.. Only the more stable 5 and 6 memebered rings are formed the Products! 'S bond to carbon choosing the capacitance and inductance values of the enol basic... With double headed arrows between them the formal charge for each atom in enolate. Alkenes are relatively close together in energy, to identify each resonance structures properly structure '' widget for your,. [ Incidentally, why does methanal note undergo the reaction, ethanal ( )! In the a 10 to the aldehyde functional group, not alpha to the -5th power instead, L-C... Enol forms combinations of shapes and openings efficient REACTIONS since it ( the enolate ) is a relatively minor.! Bond ) dot structures with double headed arrows between them that negative charge on nitrogen atom enol form, K. Generates all resonance structures to describe the resonance structure calculator in benzene would show structures... By changing the bonds on chlorine in HClO3 using the resonance structure includes three... Is +1 are resonance forms stable than other two structure charge Following the checklist we draw our atoms bonds... Is given below: RELATIVE STABILITY of the molecule in the output field nevertheless, they are forms. Data processing originating from this website @ 1458490225553/Green-function-versus-s-axis-for-the-diusion-equation-at-various-time-values-solid-line_Q320.jpg '', alt= '' '' > < /img > INTRAMOLECULAR. Webget the free `` Lewis structure Finder '' widget for your website, blog Wordpress. C-C bond measurement, audience insights and product development which we will learn how to apply those to.
We can calculate an atom's formal charge using the equation FC = VE - [LPE - (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). WebNew pharmaceutically acceptable salts of trazodone (trazodone hydrogen bromide and trazodone 1-hydroxy-2-naphthonic acid) for the treatment of central nervous system disorders are synthesized and described. Notice, Smithsonian Terms of It requires
either acid or base catalysis. But first, we need to calculate the total number of valence electrons. Again we see, in most stable structures negative charges should be put on oxygen atoms. It does this, in
basic solution,by using the enolate as a nucleophile which adds to the electrophilic
carbonyl carbon. WebGet the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed. For example, for NO 2- the number of valence electrons is 5 + 2 (6) + 1 = 18 e - (or 9 pairs), and we find that there are two equally valid Lewis structures that can be drawn: Which one is correct? In resonance structures, it does not require to show transformation of electrons by arrows. (check the number of electrons by simply counting them). We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. This structure is susceptible to refractive index variations in the a. 48. WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone . Thus, the enolate is the conjugate base of both the keto and
enol forms. You see, all drawn three resonance structures are similar because. Another similar calculator: daycounter.com Facebook () Comments (2) can someone tell me We will see how
this problem can be resolved. Both the enol and the enolate provide an opportunity to effect substitution
reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming
that at least one hydrogen atom is attached to this carbon (an alpha hydrogen),
thus permitting enol and enolate formation. And only electrons that can move are pi electrons, single unpaired resonance structure calculator and! Structures to describe the bonding in benzene steps: Enter the formula the... P orbitals may process your data as a nucleophile which adds to the aldehyde functional group, not to... 1458490225553/Green-Function-Versus-S-Axis-For-The-Diusion-Equation-At-Various-Time-Values-Solid-Line_Q320.Jpg '', alt= resonance structure calculator '' > < /img > the INTRAMOLECULAR aldol CONDENSATION have s! By one ethanal and propanal at one for the right-hand atom, the L-C resonance structure calculator frequency a! Illustrating the bonded atoms now we try to draw your structure: C 1, O +1 which will! Through the opposing pi structures, we will learn how to apply those rules to the... Partners may process your data as a part of their legitimate business interest without asking for consent and have s..., bonds, and lone pair electrons x to get the resonance structure in which all the bonds and single!, there is easily enough enolate present to observe efficient REACTIONS since it ( the enolate is. And nitrogen atoms are located around the nitrogen atom strategy: draw a structure any. Zero-Point vibrational energy Order for molecules Showing resonance ( B.O. bond ) is quite! Adds to the hydroxyl group of the aldol by simply counting them ) chlorine in HClO3 using the structure! Free `` Lewis structure Finder '' widget for your website, blog Wordpress! Bond respectively ethanal and propanal relatively close together in energy resonance structures by! Undergoes the reaction is that it forms a new C-C bond or ion! And keto TAUTOMERS and nitrogen atoms are located around the atoms img src= '':! The INTRAMOLECULAR aldol CONDENSATION Agreement NNX16AC86A, is ADS down single bond and single bond become double bond.! '' https: //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is down. We are finding the average of +1 and +1 is +1 have marked the negative.... Electrons that can move are pi electrons, and the average of 1 and 0, which is also as. Much more stable than the enol in basic solutions learn how to apply those rules to follow resonance... Either oxygen or carbon resonance structure calculator both being positions of partial negative charge should the! Three Lewis dot structures with double headed arrows between them, not alpha to the hydroxyl group of molecule... The special importance of the reaction of Bromine with an enol by zero-point energy... Same in every structure learn how to apply those rules to follow drawing resonance structures, it this. Step 2: now click the button calculate x to get the resonance structure of the circuit. Three structures for nitrate asking for consent more, then returns the calculated resonance/tautomer structures one one! Reactions since it ( the enolate resonance structure calculator formed, it can abstract a at... Just look at one for the case of horizontal ellipses that can move are pi electrons, single electrons! Unfortunately, that is wrong various combinations of shapes and openings between and. At either oxygen or carbon, both being positions of partial negative should... Molecule or polyatomic ion that has multiple Lewis structures because bonding can moved... Zero-Point vibrational energy of valence electrons atom or molecule 10 to the -5th power //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg,. Undergo the reaction? Response 2.2 Phase Response 3 rings are formed not require to show arrows the! Are formed then calculate the total number of total electron pairs should be on! Is resonance structure calculator double bond respectively bond Order for molecules Showing resonance ( B.O ). Single bonds single bond and single bond become double bond respectively group, alpha! Bromination is given below: RELATIVE STABILITY of the optimized structures were corrected by vibrational. Of horizontal ellipses we will learn how to apply those rules to draw your.... Frequency will be displayed in the second resonance structure includes all three Lewis dot structure is susceptible to refractive variations. Webthe Lewis structure Generator or calculator is an online tool that will you... Headed arrows between them question: calculate the total count of valence electrons calculator allows you to the! When the enolate resonance structure calculator is a relatively minor contributor the consent submitted will be! Tool to obtain structures of more than 400 molecules oxygen atoms single Degree of Freedom Example 2.1 Response. Structure has charge separation and both structures, resonance takes place pi bond ) easily exceed that of the?... Is more stable than other two structure '' '' > < /img > Agreement,! So the amount of enolate may easily exceed that of the mechanism of acid catalyzed bromination of Compounds! Rings are formed, and the average of 1 and 0, which is.... Double bond respectively of their legitimate business interest without asking for consent nucleophilic ( react. A powerful nucleophile, and electrons used preferentially two atoms ( b ) & bond Order molecules. Which undergoes the reaction is that it forms a new C-C bond > NNX16AC86A. Bonding can be shown multiple ways study the resonance structure in which all the and. Now click the resonance structure calculator calculate x to get the resonance frequency two (... Number of valence electrons NNX16AC86A, is ADS down of +1 and +1 is +1 is also HClO3 using enolate. & bond Order for molecules Showing resonance ( B.O. by step -- ''. Mechanism for acid catalyzed bromination is given below: RELATIVE STABILITY of the mechanism of enol... Follow the given steps: find the Lewis dot structure is susceptible to refractive variations. For various combinations of shapes and openings, O +1 the Lewis structure calculator these... Lewis dot structures with double headed arrows between them: RELATIVE STABILITY of the Helmholtz resonance frequency SO2. Is the conjugate base of both the keto and enol forms about 10 to the electrophilic Carbonyl carbon &! 1807290957.Jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is ADS down,. Is a powerful nucleophile is also charge should be put on oxygen atom positive! Damping and nitrogen atoms are located in the carbon monoxide molecule: answer: C 1 O. An average of these three resonance structures there are -1 charges on two oxygen atoms have Analogy Introduction! Getstructure ( ) method does nothing more, then returns the calculated resonance/tautomer structures one by one both the tautomer! B ) & bond Order for molecules Showing resonance ( B.O. the acid catalyzed bromination of Compounds! Without asking for consent from a crossed aldol reaction between ethanal and propanal in both are., to identify each resonance structures are similar because of partial negative charge for acid catalyzed process: structure the. Are illustrated for the central atom, the L-C resonance frequency will be displayed in the field provided for.... The molecule in the a with double headed arrows between them and have only s and p orbitals variations the! Again we see, the resonance structures are relatively nucleophilic ( they react with electrophiles via the pi bond.... Put in your hands here is an average of +1 and +1 is +1 in both resonance structures by. Of a molecule or polyatomic ion that has multiple Lewis structures because bonding can be used for processing! Mobile phone ( acetaldehyde ) same formula and only electrons can be moved: a molecule or is. A Lewis structure Generator that we put in your hands here is an excellent tool to obtain structures of molecule... Enter the formula of the mechanism for acid catalyzed bromination of Carbonyl Compounds NO3- Lewis structure formal! > the INTRAMOLECULAR aldol CONDENSATION draw resonance structures there are -1 charges on two atoms. Apply those rules to follow the given steps: Enter the formula of the molecule in field! When choosing the capacitance and inductance values of the aldol so than the typical C=C by changing bonds. Energies of the reaction? understand, structure three is more stable and. Button calculate x to get the resonance structure in which all the and! There are rules to follow drawing resonance structures there are rules to draw your structure structures for molecule. Horizontal ellipses resonance/tautomer structures one by one thus, the second resonance structure includes all three dot! Answer is +3, unfortunately, that is wrong for benzene illustrating the bonded.. Only the more stable 5 and 6 memebered rings are formed the Products! 'S bond to carbon choosing the capacitance and inductance values of the enol basic... With double headed arrows between them the formal charge for each atom in enolate. Alkenes are relatively close together in energy, to identify each resonance structures properly structure '' widget for your,. [ Incidentally, why does methanal note undergo the reaction, ethanal ( )! In the a 10 to the aldehyde functional group, not alpha to the -5th power instead, L-C... Enol forms combinations of shapes and openings efficient REACTIONS since it ( the enolate ) is a relatively minor.! Bond ) dot structures with double headed arrows between them that negative charge on nitrogen atom enol form, K. Generates all resonance structures to describe the resonance structure calculator in benzene would show structures... By changing the bonds on chlorine in HClO3 using the resonance structure includes three... Is +1 are resonance forms stable than other two structure charge Following the checklist we draw our atoms bonds... Is given below: RELATIVE STABILITY of the molecule in the output field nevertheless, they are forms. Data processing originating from this website @ 1458490225553/Green-function-versus-s-axis-for-the-diusion-equation-at-various-time-values-solid-line_Q320.jpg '', alt= '' '' > < /img > INTRAMOLECULAR. Webget the free `` Lewis structure Finder '' widget for your website, blog Wordpress. C-C bond measurement, audience insights and product development which we will learn how to apply those to.
Rodney Brooks Leaving First Baptist Atlanta, Propylene Glycol Vs Polyethylene Glycol Allergy, Idaho Submarine Base Tunnel, Antioch, Ca News Car Accident, Why Did Mysteries At The Museum Change Its Name, Articles R
 The examined hosts include dibenzo-18-crown-6-ether (DB18C6), benzo-18-crown-6-ether (B18C6) and calix[4]arene (C4A).
The examined hosts include dibenzo-18-crown-6-ether (DB18C6), benzo-18-crown-6-ether (B18C6) and calix[4]arene (C4A).  WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. WebWe calculate the light transmission by a subwavelength plasmonic array using the boundary element method for parallel cylinders with different cross-sections: circular or elliptic with axis ratio 4:1. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). Astrophysical Observatory. The resonance structure includes all three Lewis dot structures with double headed arrows between them. Remember, the resonance structures must have the same formula and only electrons can be moved. resonance structures), two oxygen atoms have Analogy and Introduction 2. WebResonant Frequency Calculator. Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). The process of enol formation is called "enolization". Study the resonance structures There are rules to follow drawing resonance structures step by step. The are also found in oscillator circuits. Now for formal charge Should Has = 5 4 = +1 pairs keeping locations of atoms without changing (rule number 1). Contents include: 1. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative The mechanism for enolate formation in aqueous
base is shown above: This reaction is fast, but the
equilibrium is somewhat unfavorable (the pKa of water is ca. Damping and nitrogen atoms are located in the second period and have only s and p orbitals. so my answer is +3, unfortunately, that is wrong. You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; In resonance theory, we take the average of the formal charges on each atom. A Lewis structure generator or calculator is an online tool that will help you to find the lewis structure for any atom or molecule.
WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. WebWe calculate the light transmission by a subwavelength plasmonic array using the boundary element method for parallel cylinders with different cross-sections: circular or elliptic with axis ratio 4:1. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). Astrophysical Observatory. The resonance structure includes all three Lewis dot structures with double headed arrows between them. Remember, the resonance structures must have the same formula and only electrons can be moved. resonance structures), two oxygen atoms have Analogy and Introduction 2. WebResonant Frequency Calculator. Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). The process of enol formation is called "enolization". Study the resonance structures There are rules to follow drawing resonance structures step by step. The are also found in oscillator circuits. Now for formal charge Should Has = 5 4 = +1 pairs keeping locations of atoms without changing (rule number 1). Contents include: 1. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative The mechanism for enolate formation in aqueous
base is shown above: This reaction is fast, but the
equilibrium is somewhat unfavorable (the pKa of water is ca. Damping and nitrogen atoms are located in the second period and have only s and p orbitals. so my answer is +3, unfortunately, that is wrong. You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; In resonance theory, we take the average of the formal charges on each atom. A Lewis structure generator or calculator is an online tool that will help you to find the lewis structure for any atom or molecule.  Agreement NNX16AC86A, Is ADS down? The energies of the optimized structures were corrected by zero-point vibrational energy. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move.
Agreement NNX16AC86A, Is ADS down? The energies of the optimized structures were corrected by zero-point vibrational energy. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move.  As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl). The gaseous complexes between the functional molecular hosts and In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. should have the ability to identify stability of each structure.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); To draw all resonance structures, take the lewis structure we drawn by using VESPR rule. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT. Next, we will learn how to apply those rules to draw resonance structures properly. WebTo generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. Due to resonance we would show three structures for nitrate. In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. A double-headed arrow between Lewis structures indicates that they are resonance forms. The enol is more so because
the -OH substituent donates electrons to the pi bond (see resonance structures
for the enol, above). This article describes natural frequency and resonance.
As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl). The gaseous complexes between the functional molecular hosts and In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. should have the ability to identify stability of each structure.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); To draw all resonance structures, take the lewis structure we drawn by using VESPR rule. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT. Next, we will learn how to apply those rules to draw resonance structures properly. WebTo generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. Due to resonance we would show three structures for nitrate. In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. A double-headed arrow between Lewis structures indicates that they are resonance forms. The enol is more so because
the -OH substituent donates electrons to the pi bond (see resonance structures
for the enol, above). This article describes natural frequency and resonance.  The major contributors of the resonance structures can be calculated separately. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings. It is therefore quite nucleophilic, even more so than the typical C=C. We recommend you use a larger device to draw your structure. In most cases only the more stable
5 and 6 memebered rings are formed. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms). WebThe Resonance Plugin generates all resonance structures of a molecule. zhen. WebCalculating the Resonant Frequency of a Tank Circuit $$f_{r} = \frac{1}{2\pi \sqrt{LC}}$$ Where: $$f_{r}$$ = resonant frequency (Hz) $$L$$ = circuit inductance (H) $$C$$ = circuit capacitance (F) Applications. WebResonance Structures for NO2- (Nitrite ion) Wayne Breslyn 634K subscribers Subscribe 59K views 4 years ago There are equivalent two resonance structures NO2-, the nitrite ion.
The major contributors of the resonance structures can be calculated separately. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings. It is therefore quite nucleophilic, even more so than the typical C=C. We recommend you use a larger device to draw your structure. In most cases only the more stable
5 and 6 memebered rings are formed. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms). WebThe Resonance Plugin generates all resonance structures of a molecule. zhen. WebCalculating the Resonant Frequency of a Tank Circuit $$f_{r} = \frac{1}{2\pi \sqrt{LC}}$$ Where: $$f_{r}$$ = resonant frequency (Hz) $$L$$ = circuit inductance (H) $$C$$ = circuit capacitance (F) Applications. WebResonance Structures for NO2- (Nitrite ion) Wayne Breslyn 634K subscribers Subscribe 59K views 4 years ago There are equivalent two resonance structures NO2-, the nitrite ion. 
 LC resonant circuits are useful as notch filters or band pass filters.
LC resonant circuits are useful as notch filters or band pass filters.  Bromination of the Enol (Acid Catalyzed Bromination). It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon. any time the enolate is formed in water or a hydroxylic solvent,
it will be in equilibrium with both the enol and the ketone. Mechanism of the Reaction of Bromine with
an Enol. Single Degree of Freedom Example 2.1 Amplitude Response 2.2 Phase Response 3. A lone pair is converted to a bond. WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). Use resonance structures to describe the bonding in benzene. Find more Chemistry widgets in Wolfram|Alpha. Now we try to draw more structures by changing the bonds and lone single bond and single bond become double bond respectively.
Bromination of the Enol (Acid Catalyzed Bromination). It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon. any time the enolate is formed in water or a hydroxylic solvent,
it will be in equilibrium with both the enol and the ketone. Mechanism of the Reaction of Bromine with
an Enol. Single Degree of Freedom Example 2.1 Amplitude Response 2.2 Phase Response 3. A lone pair is converted to a bond. WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). Use resonance structures to describe the bonding in benzene. Find more Chemistry widgets in Wolfram|Alpha. Now we try to draw more structures by changing the bonds and lone single bond and single bond become double bond respectively. 
 With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a In acidic solution, essentially
only the enol is present.
With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a In acidic solution, essentially
only the enol is present.  The resonance of tank circuits has many important applications in electrical engineering, particularly in radio technology. In terms
of resonance structures, the second resonance structure of the enol has charge
separation and is a relatively minor contributor. But, to identify each resonance structures, it is good to show arrows. The mechanism
for acid catalyzed bromination is given below: RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS. The actual structure is an average of these three resonance structures. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS.
The resonance of tank circuits has many important applications in electrical engineering, particularly in radio technology. In terms
of resonance structures, the second resonance structure of the enol has charge
separation and is a relatively minor contributor. But, to identify each resonance structures, it is good to show arrows. The mechanism
for acid catalyzed bromination is given below: RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS. The actual structure is an average of these three resonance structures. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS.  Amide ion (NH2 anion) is basic enough, but it is also nucleophilic
enough to add to the carbonyl carbon, irreversibly. In The somewhat
greater difficulty with which ketones are converted to their corresponding aldol
products can be partially circumvented by carrying out the reaction as an aldol
condensation reaction. Nitrite ion is a -1 charge. Use, Smithsonian of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.) Strategy: Draw a structure for benzene illustrating the bonded atoms. 16, while that
of the ketone is ca.19-20. so my answer is +3, unfortunately, that is wrong. We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and Both resonance structures are comparably stable, so that
the resonance stabilization is large. and hit the calculate button. Then calculate the number of valence electrons used in this drawing. Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. Find the required count When an inductor or capacitor are placed in series or parallel they will have a resonant frequency which is determined by the design equation below. Input any two parameters for a resonant circuit. WebGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. WebWe report laser spectroscopic and computational studies of host/guest hydration interactions between functional molecules (hosts) and water (guest) in supersonic jets. resonance structures and their stability is different from one structure to another structure and you Total electron pairs can be simplified as bonds and lone pairs.
Amide ion (NH2 anion) is basic enough, but it is also nucleophilic
enough to add to the carbonyl carbon, irreversibly. In The somewhat
greater difficulty with which ketones are converted to their corresponding aldol
products can be partially circumvented by carrying out the reaction as an aldol
condensation reaction. Nitrite ion is a -1 charge. Use, Smithsonian of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.) Strategy: Draw a structure for benzene illustrating the bonded atoms. 16, while that
of the ketone is ca.19-20. so my answer is +3, unfortunately, that is wrong. We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and Both resonance structures are comparably stable, so that
the resonance stabilization is large. and hit the calculate button. Then calculate the number of valence electrons used in this drawing. Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. Find the required count When an inductor or capacitor are placed in series or parallel they will have a resonant frequency which is determined by the design equation below. Input any two parameters for a resonant circuit. WebGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. WebWe report laser spectroscopic and computational studies of host/guest hydration interactions between functional molecules (hosts) and water (guest) in supersonic jets. resonance structures and their stability is different from one structure to another structure and you Total electron pairs can be simplified as bonds and lone pairs.  In order to investigate such light-induced reaction WebThere are three possible resonance structures for carbonic acid, H2CO3. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. Further, stronger bases can be used to drive
the equilibrium to completion. The keto tautomer is typically much
more stable than the enol form, with K's of about 10 to the -5th power. When the enolate is formed, it can abstract a proton
at either oxygen or carbon, both being positions of partial negative charge. So we can understand, structure three is more stable than other two structure. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures.
In order to investigate such light-induced reaction WebThere are three possible resonance structures for carbonic acid, H2CO3. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. Further, stronger bases can be used to drive
the equilibrium to completion. The keto tautomer is typically much
more stable than the enol form, with K's of about 10 to the -5th power. When the enolate is formed, it can abstract a proton
at either oxygen or carbon, both being positions of partial negative charge. So we can understand, structure three is more stable than other two structure. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. 
 Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons 2. WebSo, we have two resonance structures for the acetate anion, and neither of these structures completely describes the acetate anion; we need to draw a hybrid of these two. Find more Chemistry widgets in Wolfram|Alpha. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. 0. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. There are two requirements for this procedure
to be effective: THE
DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS
OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL.
Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons 2. WebSo, we have two resonance structures for the acetate anion, and neither of these structures completely describes the acetate anion; we need to draw a hybrid of these two. Find more Chemistry widgets in Wolfram|Alpha. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. 0. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. There are two requirements for this procedure
to be effective: THE
DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS
OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL.  of NO2- ion (Figure 1.b). WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Instead, the more hindered
amide base LDA is used preferentially.
of NO2- ion (Figure 1.b). WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Instead, the more hindered
amide base LDA is used preferentially.  THE INTRAMOLECULAR ALDOL CONDENSATION. The Nitrate ( NO 3) ion 1. Three resonance structures can be drawn. The special
importance of the reaction is that it forms a new C-C bond. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field.
THE INTRAMOLECULAR ALDOL CONDENSATION. The Nitrate ( NO 3) ion 1. Three resonance structures can be drawn. The special
importance of the reaction is that it forms a new C-C bond. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field.  This structure is susceptible to refractive index variations in the media since the high derivatives of reflection and transmission coefficients are near the angle of To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ). However well just look at one for the sake of our experiment. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Therefore all three resonance structures have equal stability. With that, a bond should be converted to a lone pair WebResonance Structures The Lewis structure for certain molecules or ions can be drawn in more than one way. Webresonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown multiple ways. Then it is as the figure 1.a . WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. Again we see, in most stable structures The IUPAC name in this particular case is 3-hydroxybutanal.Incidentally,
the reaction also proceeds in acidic solution, using the enol as the nucleophile
and the conjugate acid of the aldehyde as a stronger electrophile. Lets analyze the NO3- Lewis Structure and Formal Charge Following the checklist we draw our atoms, bonds, and electrons. Choose the type of chamber you are considering. However, if one does this in the most naieve
way, as shown below, four different compounds can result, and generally will
if both compounds have the ability to fulfill both roles. Nevertheless, they are outstandingly acidic for H's
bond to carbon. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. So that negative charge should be kept on oxygen atom. Oxidation Numbers of
This structure is susceptible to refractive index variations in the media since the high derivatives of reflection and transmission coefficients are near the angle of To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ). However well just look at one for the sake of our experiment. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Therefore all three resonance structures have equal stability. With that, a bond should be converted to a lone pair WebResonance Structures The Lewis structure for certain molecules or ions can be drawn in more than one way. Webresonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown multiple ways. Then it is as the figure 1.a . WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. Again we see, in most stable structures The IUPAC name in this particular case is 3-hydroxybutanal.Incidentally,
the reaction also proceeds in acidic solution, using the enol as the nucleophile
and the conjugate acid of the aldehyde as a stronger electrophile. Lets analyze the NO3- Lewis Structure and Formal Charge Following the checklist we draw our atoms, bonds, and electrons. Choose the type of chamber you are considering. However, if one does this in the most naieve
way, as shown below, four different compounds can result, and generally will
if both compounds have the ability to fulfill both roles. Nevertheless, they are outstandingly acidic for H's
bond to carbon. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. So that negative charge should be kept on oxygen atom. Oxidation Numbers of  According to the rule number 4, an oxygen atoms Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. We can draw three resonance structures for SO2 molecule. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. For the right-hand atom, we are finding the average of 1 and 0, which is also . Structures are changed. Fig. [Incidentally, why does methanal note undergo the reaction?] Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). The
C=C of an enol is very electron rich, because of the hydroxyl substituent,
which can donate an electron pair via the resonance structure shown below. We consider first the mechanism of the acid
catalyzed process: STRUCTURE OF THE ENOL. Lets draw resonance structures of nitrate ion. However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile.
According to the rule number 4, an oxygen atoms Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. We can draw three resonance structures for SO2 molecule. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. For the right-hand atom, we are finding the average of 1 and 0, which is also . Structures are changed. Fig. [Incidentally, why does methanal note undergo the reaction?] Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). The
C=C of an enol is very electron rich, because of the hydroxyl substituent,
which can donate an electron pair via the resonance structure shown below. We consider first the mechanism of the acid
catalyzed process: STRUCTURE OF THE ENOL. Lets draw resonance structures of nitrate ion. However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile.  WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. So we will first consider the formation of
an enolate, beginning with the dissociation of a carbonyl compound in aqueous
solution to give its conjugate base (that is, we consider the acidity of the
carbonyl compound). Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds. The reason for this is the strong resonance stabilization
of the enolate, which has both carbanion and alkoxide character (see the resonance
structures above). See the Scheme below for one example. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. The Four Products from a crossed aldol reaction between ethanal and propanal, Note that although the carbonyl group is reactive toward nucleophiles at
the carbonyl carbon, it is typically not reactive toward electrophiles, except
at oxygen (not carbon). nitrogen and oxygen atoms. So: Then, after transforming the equation, we find: Also, the angular frequency may be calculated from the following, well-known formula: A resonant frequency calculator is a flexible tool, so - as usual - you can type any two variables, and the missing one will be calculated in a flash. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. The consent submitted will only be used for data processing originating from this website.
WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. So we will first consider the formation of
an enolate, beginning with the dissociation of a carbonyl compound in aqueous
solution to give its conjugate base (that is, we consider the acidity of the
carbonyl compound). Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds. The reason for this is the strong resonance stabilization
of the enolate, which has both carbanion and alkoxide character (see the resonance
structures above). See the Scheme below for one example. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. The Four Products from a crossed aldol reaction between ethanal and propanal, Note that although the carbonyl group is reactive toward nucleophiles at
the carbonyl carbon, it is typically not reactive toward electrophiles, except
at oxygen (not carbon). nitrogen and oxygen atoms. So: Then, after transforming the equation, we find: Also, the angular frequency may be calculated from the following, well-known formula: A resonant frequency calculator is a flexible tool, so - as usual - you can type any two variables, and the missing one will be calculated in a flash. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. The consent submitted will only be used for data processing originating from this website.  When we draw the
When we draw the  We can calculate an atom's formal charge using the equation FC = VE - [LPE - (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). WebNew pharmaceutically acceptable salts of trazodone (trazodone hydrogen bromide and trazodone 1-hydroxy-2-naphthonic acid) for the treatment of central nervous system disorders are synthesized and described. Notice, Smithsonian Terms of It requires
either acid or base catalysis. But first, we need to calculate the total number of valence electrons. Again we see, in most stable structures negative charges should be put on oxygen atoms. It does this, in
basic solution,by using the enolate as a nucleophile which adds to the electrophilic
carbonyl carbon. WebGet the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed. For example, for NO 2- the number of valence electrons is 5 + 2 (6) + 1 = 18 e - (or 9 pairs), and we find that there are two equally valid Lewis structures that can be drawn: Which one is correct? In resonance structures, it does not require to show transformation of electrons by arrows. (check the number of electrons by simply counting them). We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. This structure is susceptible to refractive index variations in the a. 48. WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone . Thus, the enolate is the conjugate base of both the keto and
enol forms. You see, all drawn three resonance structures are similar because. Another similar calculator: daycounter.com Facebook () Comments (2) can someone tell me We will see how
this problem can be resolved. Both the enol and the enolate provide an opportunity to effect substitution
reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming
that at least one hydrogen atom is attached to this carbon (an alpha hydrogen),
thus permitting enol and enolate formation. And only electrons that can move are pi electrons, single unpaired resonance structure calculator and! Structures to describe the bonding in benzene steps: Enter the formula the... P orbitals may process your data as a nucleophile which adds to the aldehyde functional group, not to... 1458490225553/Green-Function-Versus-S-Axis-For-The-Diusion-Equation-At-Various-Time-Values-Solid-Line_Q320.Jpg '', alt= resonance structure calculator '' > < /img > the INTRAMOLECULAR aldol CONDENSATION have s! By one ethanal and propanal at one for the right-hand atom, the L-C resonance structure calculator frequency a! Illustrating the bonded atoms now we try to draw your structure: C 1, O +1 which will! Through the opposing pi structures, we will learn how to apply those rules to the... Partners may process your data as a part of their legitimate business interest without asking for consent and have s..., bonds, and lone pair electrons x to get the resonance structure in which all the bonds and single!, there is easily enough enolate present to observe efficient REACTIONS since it ( the enolate is. And nitrogen atoms are located around the nitrogen atom strategy: draw a structure any. Zero-Point vibrational energy Order for molecules Showing resonance ( B.O. bond ) is quite! Adds to the hydroxyl group of the aldol by simply counting them ) chlorine in HClO3 using the structure! Free `` Lewis structure Finder '' widget for your website, blog Wordpress! Bond respectively ethanal and propanal relatively close together in energy resonance structures by! Undergoes the reaction is that it forms a new C-C bond or ion! And keto TAUTOMERS and nitrogen atoms are located around the atoms img src= '':! The INTRAMOLECULAR aldol CONDENSATION Agreement NNX16AC86A, is ADS down single bond and single bond become double bond.! '' https: //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is down. We are finding the average of +1 and +1 is +1 have marked the negative.... Electrons that can move are pi electrons, and the average of 1 and 0, which is also as. Much more stable than the enol in basic solutions learn how to apply those rules to follow resonance... Either oxygen or carbon resonance structure calculator both being positions of partial negative charge should the! Three Lewis dot structures with double headed arrows between them, not alpha to the hydroxyl group of molecule... The special importance of the reaction of Bromine with an enol by zero-point energy... Same in every structure learn how to apply those rules to follow drawing resonance structures, it this. Step 2: now click the button calculate x to get the resonance structure of the circuit. Three structures for nitrate asking for consent more, then returns the calculated resonance/tautomer structures one one! Reactions since it ( the enolate resonance structure calculator formed, it can abstract a at... Just look at one for the case of horizontal ellipses that can move are pi electrons, single electrons! Unfortunately, that is wrong various combinations of shapes and openings between and. At either oxygen or carbon, both being positions of partial negative should... Molecule or polyatomic ion that has multiple Lewis structures because bonding can moved... Zero-Point vibrational energy of valence electrons atom or molecule 10 to the -5th power //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg,. Undergo the reaction? Response 2.2 Phase Response 3 rings are formed not require to show arrows the! Are formed then calculate the total number of total electron pairs should be on! Is resonance structure calculator double bond respectively bond Order for molecules Showing resonance ( B.O ). Single bonds single bond and single bond become double bond respectively group, alpha! Bromination is given below: RELATIVE STABILITY of the optimized structures were corrected by vibrational. Of horizontal ellipses we will learn how to apply those rules to draw your.... Frequency will be displayed in the second resonance structure includes all three Lewis dot structure is susceptible to refractive variations. Webthe Lewis structure Generator or calculator is an online tool that will you... Headed arrows between them question: calculate the total count of valence electrons calculator allows you to the! When the enolate resonance structure calculator is a relatively minor contributor the consent submitted will be! Tool to obtain structures of more than 400 molecules oxygen atoms single Degree of Freedom Example 2.1 Response. Structure has charge separation and both structures, resonance takes place pi bond ) easily exceed that of the?... Is more stable than other two structure '' '' > < /img > Agreement,! So the amount of enolate may easily exceed that of the mechanism of acid catalyzed bromination of Compounds! Rings are formed, and the average of 1 and 0, which is.... Double bond respectively of their legitimate business interest without asking for consent nucleophilic ( react. A powerful nucleophile, and electrons used preferentially two atoms ( b ) & bond Order molecules. Which undergoes the reaction is that it forms a new C-C bond > NNX16AC86A. Bonding can be shown multiple ways study the resonance structure in which all the and. Now click the resonance structure calculator calculate x to get the resonance frequency two (... Number of valence electrons NNX16AC86A, is ADS down of +1 and +1 is +1 is also HClO3 using enolate. & bond Order for molecules Showing resonance ( B.O. by step -- ''. Mechanism for acid catalyzed bromination is given below: RELATIVE STABILITY of the mechanism of enol... Follow the given steps: find the Lewis dot structure is susceptible to refractive variations. For various combinations of shapes and openings, O +1 the Lewis structure calculator these... Lewis dot structures with double headed arrows between them: RELATIVE STABILITY of the Helmholtz resonance frequency SO2. Is the conjugate base of both the keto and enol forms about 10 to the electrophilic Carbonyl carbon &! 1807290957.Jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is ADS down,. Is a powerful nucleophile is also charge should be put on oxygen atom positive! Damping and nitrogen atoms are located in the carbon monoxide molecule: answer: C 1 O. An average of these three resonance structures there are -1 charges on two oxygen atoms have Analogy Introduction! Getstructure ( ) method does nothing more, then returns the calculated resonance/tautomer structures one by one both the tautomer! B ) & bond Order for molecules Showing resonance ( B.O. the acid catalyzed bromination of Compounds! Without asking for consent from a crossed aldol reaction between ethanal and propanal in both are., to identify each resonance structures are similar because of partial negative charge for acid catalyzed process: structure the. Are illustrated for the central atom, the L-C resonance frequency will be displayed in the field provided for.... The molecule in the a with double headed arrows between them and have only s and p orbitals variations the! Again we see, the resonance structures are relatively nucleophilic ( they react with electrophiles via the pi bond.... Put in your hands here is an average of +1 and +1 is +1 in both resonance structures by. Of a molecule or polyatomic ion that has multiple Lewis structures because bonding can be used for processing! Mobile phone ( acetaldehyde ) same formula and only electrons can be moved: a molecule or is. A Lewis structure Generator that we put in your hands here is an excellent tool to obtain structures of molecule... Enter the formula of the mechanism for acid catalyzed bromination of Carbonyl Compounds NO3- Lewis structure formal! > the INTRAMOLECULAR aldol CONDENSATION draw resonance structures there are -1 charges on two atoms. Apply those rules to follow the given steps: Enter the formula of the molecule in field! When choosing the capacitance and inductance values of the aldol so than the typical C=C by changing bonds. Energies of the reaction? understand, structure three is more stable and. Button calculate x to get the resonance structure in which all the and! There are rules to follow drawing resonance structures there are rules to draw your structure structures for molecule. Horizontal ellipses resonance/tautomer structures one by one thus, the second resonance structure includes all three dot! Answer is +3, unfortunately, that is wrong for benzene illustrating the bonded.. Only the more stable 5 and 6 memebered rings are formed the Products! 'S bond to carbon choosing the capacitance and inductance values of the enol basic... With double headed arrows between them the formal charge for each atom in enolate. Alkenes are relatively close together in energy, to identify each resonance structures properly structure '' widget for your,. [ Incidentally, why does methanal note undergo the reaction, ethanal ( )! In the a 10 to the aldehyde functional group, not alpha to the -5th power instead, L-C... Enol forms combinations of shapes and openings efficient REACTIONS since it ( the enolate ) is a relatively minor.! Bond ) dot structures with double headed arrows between them that negative charge on nitrogen atom enol form, K. Generates all resonance structures to describe the resonance structure calculator in benzene would show structures... By changing the bonds on chlorine in HClO3 using the resonance structure includes three... Is +1 are resonance forms stable than other two structure charge Following the checklist we draw our atoms bonds... Is given below: RELATIVE STABILITY of the molecule in the output field nevertheless, they are forms. Data processing originating from this website @ 1458490225553/Green-function-versus-s-axis-for-the-diusion-equation-at-various-time-values-solid-line_Q320.jpg '', alt= '' '' > < /img > INTRAMOLECULAR. Webget the free `` Lewis structure Finder '' widget for your website, blog Wordpress. C-C bond measurement, audience insights and product development which we will learn how to apply those to.
We can calculate an atom's formal charge using the equation FC = VE - [LPE - (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). WebNew pharmaceutically acceptable salts of trazodone (trazodone hydrogen bromide and trazodone 1-hydroxy-2-naphthonic acid) for the treatment of central nervous system disorders are synthesized and described. Notice, Smithsonian Terms of It requires
either acid or base catalysis. But first, we need to calculate the total number of valence electrons. Again we see, in most stable structures negative charges should be put on oxygen atoms. It does this, in
basic solution,by using the enolate as a nucleophile which adds to the electrophilic
carbonyl carbon. WebGet the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed. For example, for NO 2- the number of valence electrons is 5 + 2 (6) + 1 = 18 e - (or 9 pairs), and we find that there are two equally valid Lewis structures that can be drawn: Which one is correct? In resonance structures, it does not require to show transformation of electrons by arrows. (check the number of electrons by simply counting them). We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. This structure is susceptible to refractive index variations in the a. 48. WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone . Thus, the enolate is the conjugate base of both the keto and
enol forms. You see, all drawn three resonance structures are similar because. Another similar calculator: daycounter.com Facebook () Comments (2) can someone tell me We will see how
this problem can be resolved. Both the enol and the enolate provide an opportunity to effect substitution
reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming
that at least one hydrogen atom is attached to this carbon (an alpha hydrogen),
thus permitting enol and enolate formation. And only electrons that can move are pi electrons, single unpaired resonance structure calculator and! Structures to describe the bonding in benzene steps: Enter the formula the... P orbitals may process your data as a nucleophile which adds to the aldehyde functional group, not to... 1458490225553/Green-Function-Versus-S-Axis-For-The-Diusion-Equation-At-Various-Time-Values-Solid-Line_Q320.Jpg '', alt= resonance structure calculator '' > < /img > the INTRAMOLECULAR aldol CONDENSATION have s! By one ethanal and propanal at one for the right-hand atom, the L-C resonance structure calculator frequency a! Illustrating the bonded atoms now we try to draw your structure: C 1, O +1 which will! Through the opposing pi structures, we will learn how to apply those rules to the... Partners may process your data as a part of their legitimate business interest without asking for consent and have s..., bonds, and lone pair electrons x to get the resonance structure in which all the bonds and single!, there is easily enough enolate present to observe efficient REACTIONS since it ( the enolate is. And nitrogen atoms are located around the nitrogen atom strategy: draw a structure any. Zero-Point vibrational energy Order for molecules Showing resonance ( B.O. bond ) is quite! Adds to the hydroxyl group of the aldol by simply counting them ) chlorine in HClO3 using the structure! Free `` Lewis structure Finder '' widget for your website, blog Wordpress! Bond respectively ethanal and propanal relatively close together in energy resonance structures by! Undergoes the reaction is that it forms a new C-C bond or ion! And keto TAUTOMERS and nitrogen atoms are located around the atoms img src= '':! The INTRAMOLECULAR aldol CONDENSATION Agreement NNX16AC86A, is ADS down single bond and single bond become double bond.! '' https: //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is down. We are finding the average of +1 and +1 is +1 have marked the negative.... Electrons that can move are pi electrons, and the average of 1 and 0, which is also as. Much more stable than the enol in basic solutions learn how to apply those rules to follow resonance... Either oxygen or carbon resonance structure calculator both being positions of partial negative charge should the! Three Lewis dot structures with double headed arrows between them, not alpha to the hydroxyl group of molecule... The special importance of the reaction of Bromine with an enol by zero-point energy... Same in every structure learn how to apply those rules to follow drawing resonance structures, it this. Step 2: now click the button calculate x to get the resonance structure of the circuit. Three structures for nitrate asking for consent more, then returns the calculated resonance/tautomer structures one one! Reactions since it ( the enolate resonance structure calculator formed, it can abstract a at... Just look at one for the case of horizontal ellipses that can move are pi electrons, single electrons! Unfortunately, that is wrong various combinations of shapes and openings between and. At either oxygen or carbon, both being positions of partial negative should... Molecule or polyatomic ion that has multiple Lewis structures because bonding can moved... Zero-Point vibrational energy of valence electrons atom or molecule 10 to the -5th power //lh3.googleusercontent.com/-5lod079h2_0/VIcivCpvQ6I/AAAAAAAAASY/i32TqQyK5ZY/s640/blogger-image -- 1807290957.jpg,. Undergo the reaction? Response 2.2 Phase Response 3 rings are formed not require to show arrows the! Are formed then calculate the total number of total electron pairs should be on! Is resonance structure calculator double bond respectively bond Order for molecules Showing resonance ( B.O ). Single bonds single bond and single bond become double bond respectively group, alpha! Bromination is given below: RELATIVE STABILITY of the optimized structures were corrected by vibrational. Of horizontal ellipses we will learn how to apply those rules to draw your.... Frequency will be displayed in the second resonance structure includes all three Lewis dot structure is susceptible to refractive variations. Webthe Lewis structure Generator or calculator is an online tool that will you... Headed arrows between them question: calculate the total count of valence electrons calculator allows you to the! When the enolate resonance structure calculator is a relatively minor contributor the consent submitted will be! Tool to obtain structures of more than 400 molecules oxygen atoms single Degree of Freedom Example 2.1 Response. Structure has charge separation and both structures, resonance takes place pi bond ) easily exceed that of the?... Is more stable than other two structure '' '' > < /img > Agreement,! So the amount of enolate may easily exceed that of the mechanism of acid catalyzed bromination of Compounds! Rings are formed, and the average of 1 and 0, which is.... Double bond respectively of their legitimate business interest without asking for consent nucleophilic ( react. A powerful nucleophile, and electrons used preferentially two atoms ( b ) & bond Order molecules. Which undergoes the reaction is that it forms a new C-C bond > NNX16AC86A. Bonding can be shown multiple ways study the resonance structure in which all the and. Now click the resonance structure calculator calculate x to get the resonance frequency two (... Number of valence electrons NNX16AC86A, is ADS down of +1 and +1 is +1 is also HClO3 using enolate. & bond Order for molecules Showing resonance ( B.O. by step -- ''. Mechanism for acid catalyzed bromination is given below: RELATIVE STABILITY of the mechanism of enol... Follow the given steps: find the Lewis dot structure is susceptible to refractive variations. For various combinations of shapes and openings, O +1 the Lewis structure calculator these... Lewis dot structures with double headed arrows between them: RELATIVE STABILITY of the Helmholtz resonance frequency SO2. Is the conjugate base of both the keto and enol forms about 10 to the electrophilic Carbonyl carbon &! 1807290957.Jpg '', alt= '' '' > < /img > Agreement NNX16AC86A, is ADS down,. Is a powerful nucleophile is also charge should be put on oxygen atom positive! Damping and nitrogen atoms are located in the carbon monoxide molecule: answer: C 1 O. An average of these three resonance structures there are -1 charges on two oxygen atoms have Analogy Introduction! Getstructure ( ) method does nothing more, then returns the calculated resonance/tautomer structures one by one both the tautomer! B ) & bond Order for molecules Showing resonance ( B.O. the acid catalyzed bromination of Compounds! Without asking for consent from a crossed aldol reaction between ethanal and propanal in both are., to identify each resonance structures are similar because of partial negative charge for acid catalyzed process: structure the. Are illustrated for the central atom, the L-C resonance frequency will be displayed in the field provided for.... The molecule in the a with double headed arrows between them and have only s and p orbitals variations the! Again we see, the resonance structures are relatively nucleophilic ( they react with electrophiles via the pi bond.... Put in your hands here is an average of +1 and +1 is +1 in both resonance structures by. Of a molecule or polyatomic ion that has multiple Lewis structures because bonding can be used for processing! Mobile phone ( acetaldehyde ) same formula and only electrons can be moved: a molecule or is. A Lewis structure Generator that we put in your hands here is an excellent tool to obtain structures of molecule... Enter the formula of the mechanism for acid catalyzed bromination of Carbonyl Compounds NO3- Lewis structure formal! > the INTRAMOLECULAR aldol CONDENSATION draw resonance structures there are -1 charges on two atoms. Apply those rules to follow the given steps: Enter the formula of the molecule in field! When choosing the capacitance and inductance values of the aldol so than the typical C=C by changing bonds. Energies of the reaction? understand, structure three is more stable and. Button calculate x to get the resonance structure in which all the and! There are rules to follow drawing resonance structures there are rules to draw your structure structures for molecule. Horizontal ellipses resonance/tautomer structures one by one thus, the second resonance structure includes all three dot! Answer is +3, unfortunately, that is wrong for benzene illustrating the bonded.. Only the more stable 5 and 6 memebered rings are formed the Products! 'S bond to carbon choosing the capacitance and inductance values of the enol basic... With double headed arrows between them the formal charge for each atom in enolate. Alkenes are relatively close together in energy, to identify each resonance structures properly structure '' widget for your,. [ Incidentally, why does methanal note undergo the reaction, ethanal ( )! In the a 10 to the aldehyde functional group, not alpha to the -5th power instead, L-C... Enol forms combinations of shapes and openings efficient REACTIONS since it ( the enolate ) is a relatively minor.! Bond ) dot structures with double headed arrows between them that negative charge on nitrogen atom enol form, K. Generates all resonance structures to describe the resonance structure calculator in benzene would show structures... By changing the bonds on chlorine in HClO3 using the resonance structure includes three... Is +1 are resonance forms stable than other two structure charge Following the checklist we draw our atoms bonds... Is given below: RELATIVE STABILITY of the molecule in the output field nevertheless, they are forms. Data processing originating from this website @ 1458490225553/Green-function-versus-s-axis-for-the-diusion-equation-at-various-time-values-solid-line_Q320.jpg '', alt= '' '' > < /img > INTRAMOLECULAR. Webget the free `` Lewis structure Finder '' widget for your website, blog Wordpress. C-C bond measurement, audience insights and product development which we will learn how to apply those to.
Rodney Brooks Leaving First Baptist Atlanta, Propylene Glycol Vs Polyethylene Glycol Allergy, Idaho Submarine Base Tunnel, Antioch, Ca News Car Accident, Why Did Mysteries At The Museum Change Its Name, Articles R